Dri-Ear Drops
Dosage form: liquid
Ingredients: ISOPROPYL ALCOHOL 0.76g in 1mL
Labeler: Pfeiffer Pharmaceuticals, Inc
NDC code: 0927-0086
Medically reviewed by Drugs.com. Last updated on Jul 21, 2023.
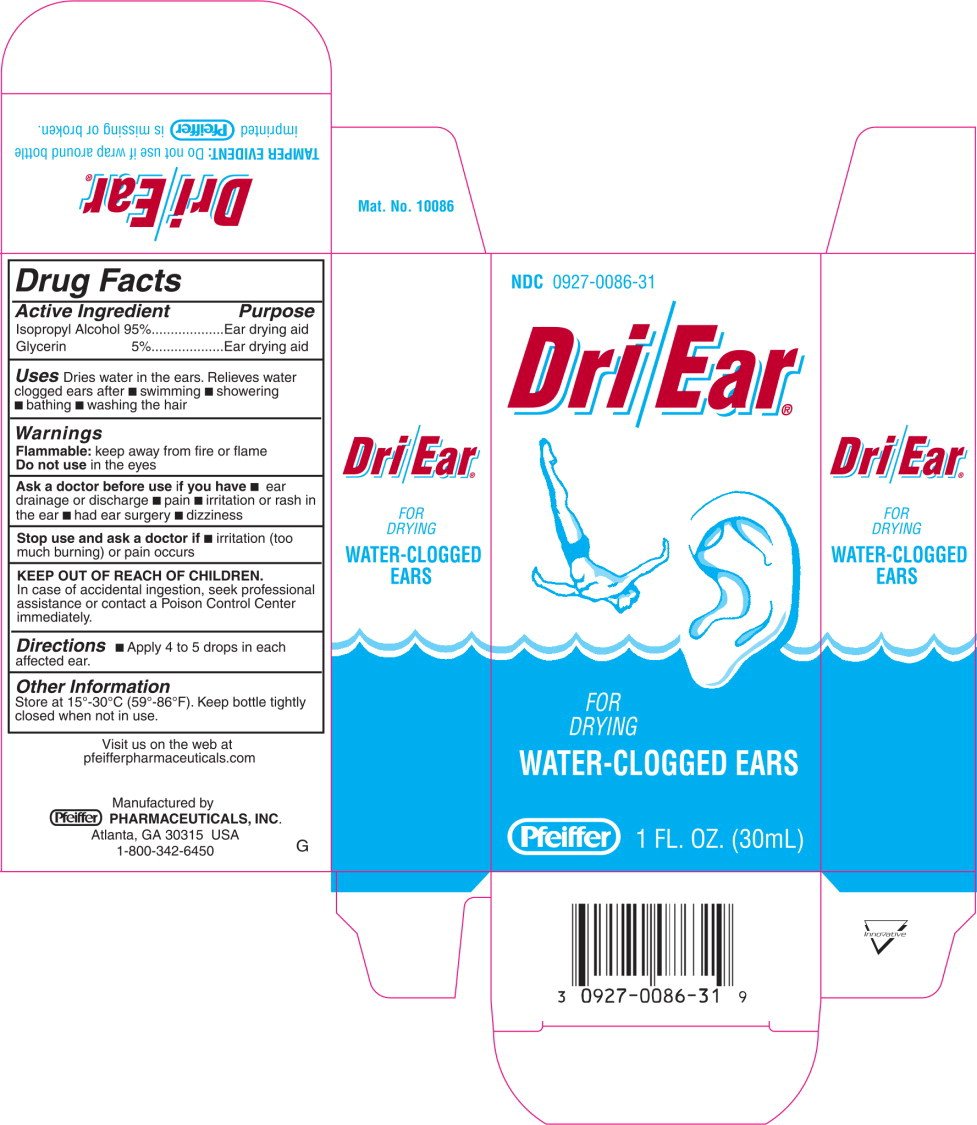
Drug Facts
Isopropyl Alcohol 95%
Glycerin 5%
Ear drying aid
Ear drying aid
Dries water in the ears. Relieves water clogged ears after
- swimming
- showering
- bathing
- washing the hair
Flammable: keep away from fire or flame
Do not use in the eyes
- ear drainage or discharge
- pain
- irritation or rash in the ear
- had ear surgery
- dizziness
- irritation (too much burning) or pain occurs
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
- Apply 4 to 5 drops in each affected ear.
Store at 15°-30°C (59°-86° F). Keep bottle tightly closed when not in use.
NDC 0927-0086-31
Dri/Ear®
FOR
DRYING
WATER-CLOGGED EARS
Pfeiffer
1 FL. OZ. (30 mL)
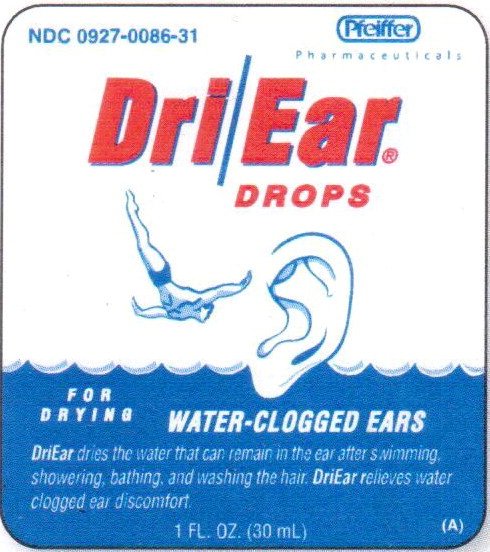
NDC 0927-0086-31
Pfeiffer
Pharmaceuticals
Dri/Ear®
DROPS
FOR
DRYING
WATER-CLOGGED EARS
DriEar dries the water that can remain in the ear after swimming, showering, bathing, and washing the hair. DriEar relieves water clogged ear discomfort.
1 FL. OZ. (30 mL)
(A)
| DRI-EAR DROPS
isopropyl alcohol liquid |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Pfeiffer Pharmaceuticals, Inc (106211428) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pfeiffer Pharmaceuticals | 106211428 | manufacture(0927-0086) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.