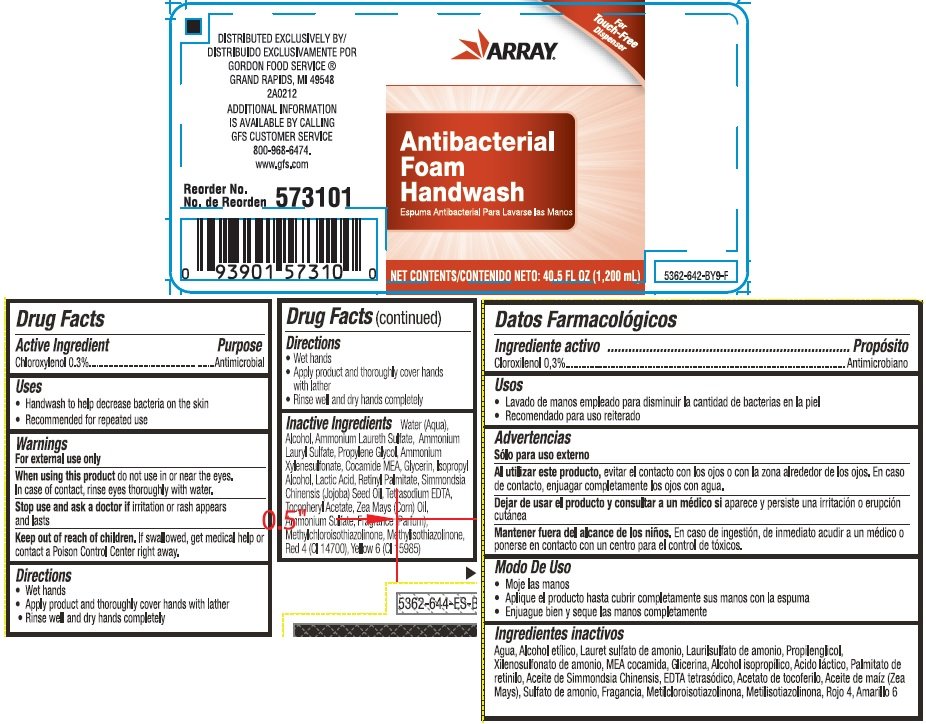
ARRAY Anitbacterial Foam Handwash
Dosage form: liquid
Ingredients: CHLOROXYLENOL 0.003mg in 1mL
Labeler: Gordon Food Service, Inc.
NDC code: 52220-080
Medically reviewed by Drugs.com. Last updated on Sep 5, 2023.
Chloroxylenol 0.3%
Antimicrobial
- Handwash to help decrease bacteria on the skin
- Recommended for repeated use
For external use only
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash appears and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Wet hands
- Apply a small amount of product and work into a lather
- Rinse well and dry hands completely
Water (Aqua), Alcohol, Ammonium Laureth Sulfate, Ammonium Lauryl Sulfate, Propylene Glycol, Ammonium Xylenesulfonate, Cocamide MEA, Glycerin, Isopropyl Alcohol, Lactic Acid, Retinyl Palmitate, Simmondsia Chinensis (Jojoba) Seed Oil, Tetrasodium EDTA, Tocopheryl Acetate, Zea Mays (Corn) Oil, Ammonium Sulfate, Fragrance (Parfum), Methylchloroisothiazolinone, Methylisothiazolinone, Red 4 (CI 14700), Yellow 6 (CI 15985)
| ARRAY ANITBACTERIAL FOAM HANDWASH
chloroxylenol liquid |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Gordon Food Service, Inc. (006409908) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.