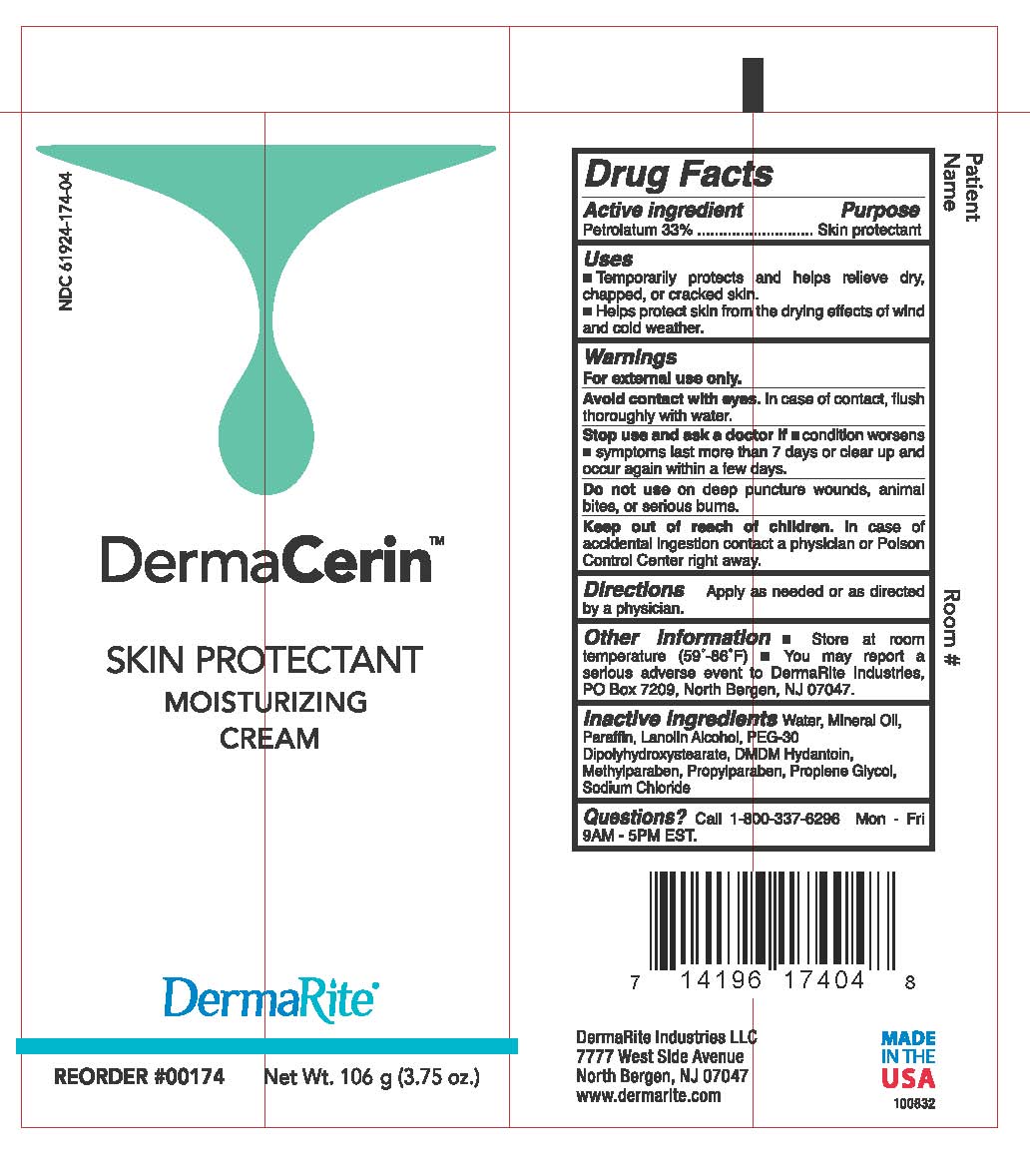
DermaCerin
Dosage form: ointment
Ingredients: PETROLATUM 33g in 100g
Labeler: DermaRite Industries, LLC
NDC code: 61924-174
Medically reviewed by Drugs.com. Last updated on Jan 8, 2024.
Petrolatum 33%
Skin Protectant
- Temporarily protects and helps relieve dry, chapped, or cracked skin.
- Helps protect skin from the drying effects of wind and cold weather.
For external use only.
Avoid contact with eyes. In case of contact, flush thoroughly with water
Stop and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days.
Do not use on deep or puncture wounds, animal bites, or serious wounds.
Keep out of reach of children.
In case of accidental ingestion contact a Physician or Poison Control Center right away.
Apply as needed or as directed by a physician.
- Store at room temperature (59º- 86ºF)
- You may report a serious adverse event to DermaRite Industries, PO Box 7209, North Bergen, NJ 07047.
Water, Mineral Oil, Paraffin, Lanolin Alcohol, PEG-30 Dipolhydroxystearate, DMDM Hydantoin, Methylparaben, Propylparaben, Propylene Glycol, Sodium Chloride
Call 800-337-6296 Mon - Fri 9AM - 5PM EST.
| DERMACERIN
skin protectant ointment |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - DermaRite Industries, LLC (883925562) |
| Registrant - DermaRite Industries, LLC (883925562) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DermaRite Industries, LLC | 883925562 | manufacture(61924-174) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.