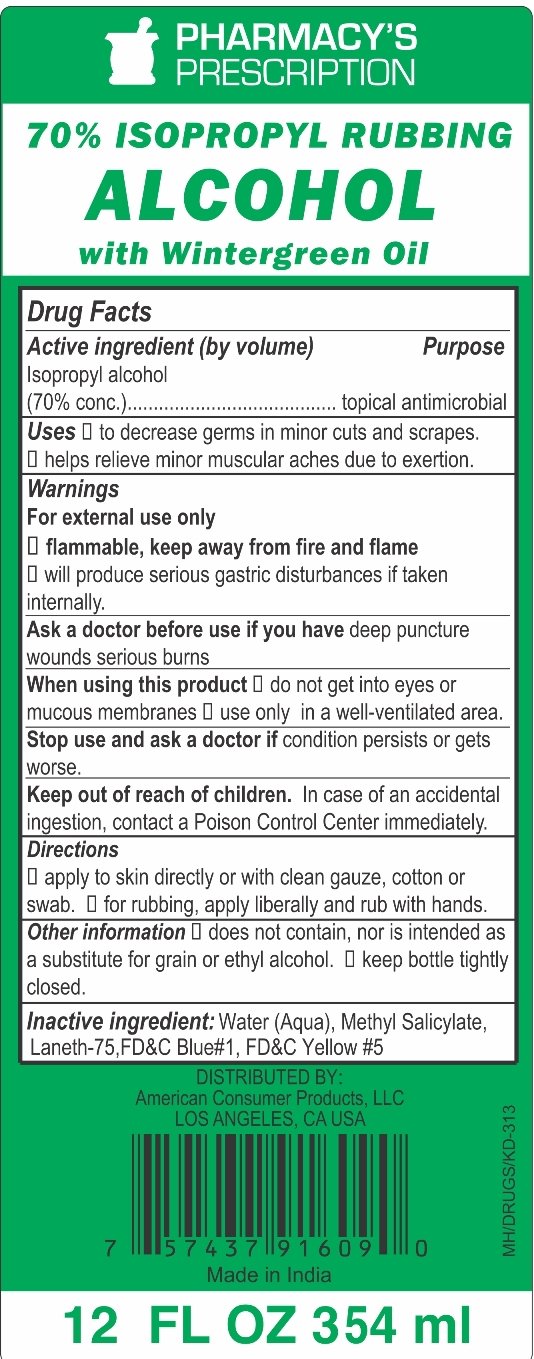
70% Isopropyl Rubbing Alcohol with Wintergreen
Dosage form: liquid
Ingredients: ISOPROPYL ALCOHOL 70mL in 100mL
Labeler: AMERICAN CONSUMER PRODUCTS LLC
NDC code: 18027-005
Medically reviewed by Drugs.com. Last updated on Jul 10, 2023.
Active Ingredient (by volume)
Isopropyl alcohol (70% conc.)
Purpose
Topical Antimicrobial
Uses
- decrease germs in minor cuts and scrapes.
- helps relieve minor muscular aches due to exertion.
Warnings
For external use only
- flammable, keep away from fire and flame
- will produce serious gastric disturbances if taken internally.
Ask a doctor before use if you have deep puncture wounds serious burns
When using this product
- do not get into eyes or mucous membranes
- use only in a well-ventilated area
Stop use and ask a doctor if condition persists or gets worse.
Keep out of reach of children. In case of an accidental ingestion, contact a Poison Control Center immediately.
Directions
- apply to skin directly or with clean gauze, cotton or swab
- for rubbing, apply liberally and rub with hands
Other information
- does not contain, nor is intended as a substitute for grain or ethyl alcohol
- keep bottle tightly closed
Inactive Ingredients
Water (Aqua), Methyl Salicylate, Laneth-75, FD&C Blue #1, FD&C Yellow #5
| 70% ISOPROPYL RUBBING ALCOHOL WITH WINTERGREEN
isopropyl alcohol liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - AMERICAN CONSUMER PRODUCTS LLC (858427334) |
| Registrant - Anicare Pharmaceuticals Pvt. Ltd (916837425) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Anicare Pharmaceuticals Pvt. Ltd | 916837425 | manufacture(18027-005) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.