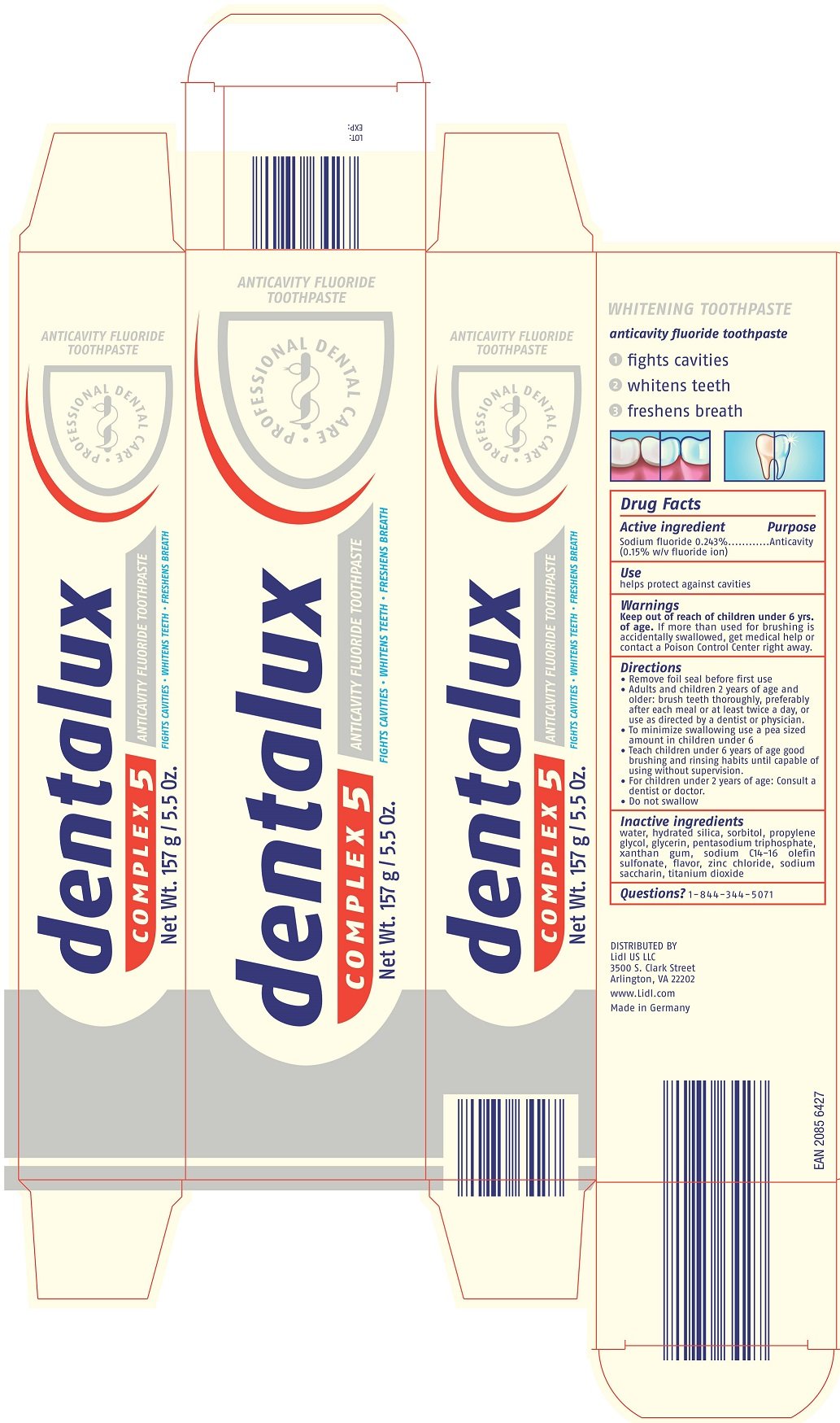
DENTALUX COMPLEX 5
Dosage form: paste
Ingredients: SODIUM FLUORIDE 0.243g in 100g
Labeler: LIDL US, LLC
NDC code: 71141-113
Medically reviewed by Drugs.com. Last updated on Oct 30, 2023.
SODIUM FLUORIDE 0.243%
(0.15% W/V FLUORIDE ION)
ANTICAVITY
HELPS PROTECT AGAINST CAVITIES
Keep out of reach of children under 6 yrs. of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
KEEP OUT OF REACH OF CHILDREN.
- Remove foil seal before first use
- Adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, or use as directed by a dentist or physician.
- To minimize swallowing use a pea sized amount in children under 6
- Teach children under 6 years of age good brushing and rinsing habits until capable of using without supervision.
- For children under 2 years of age: Consult a dentist or doctor.
- Do not swallow
water, hydrated silica, sorbitol, propylene glycol, glycerin, pentasodium triphosphate, xanthan gum, sodium C14-16 olefin sulfonate, flavor, zinc chloride, sodium saccharin, titanium dioxide
1-844-344-5071
| DENTALUX COMPLEX 5
sodium fluoride paste |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - LIDL US, LLC (079389709) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DENTAL-Kosmetik GmbH & Co. KG | 330626300 | manufacture(71141-113) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.