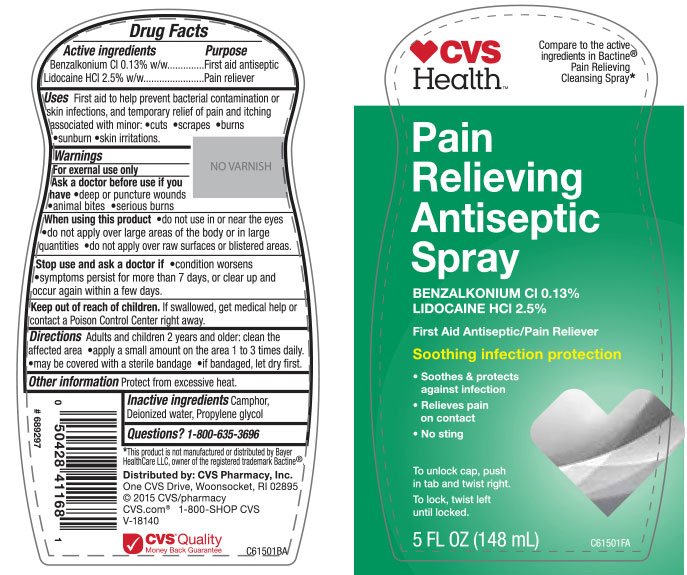
CVS Pain Relieving First Aid Antiseptic
Dosage form: spray
Ingredients: LIDOCAINE HYDROCHLORIDE 0.025g in 1g, BENZALKONIUM CHLORIDE 0.0013g in 1g
Labeler: CVS Pharmacy
NDC code: 69842-615
Medically reviewed by Drugs.com. Last updated on Dec 25, 2023.
Active Ingredients
Benzalkonium Cl 0.13% w/w
Lidocaine HCl 2.5% w/w
First aid antiseptic
Pain reliever
Firs aid to help prevent bacterial contamination or skin infections, and temporary relief of pain and itching associated with minor:
cuts
scrapes
burns
sunburns
skin irritations
For external use only
deep or puncture wounds
animal bites
serious burns
do not use near the eyes
do not apply over large areas of the body or in large quantities
do not apply over raw surfaces or blistered areas
condition worsens
symptoms persist for more than 7 days, or clear up and occur again within a few days.
If swallowed, get medical help or contact a Poison Control Center right away.
Adults and children 2 years and older:
clean the affected area
apply a small amount on the area 1 to 3 times daily.
may be covered with a sterile bandage
if bandaged, let dry first.
Protect from excessive heat.
1-800-635-3696
Camphor, Deionized Water, Propylene Glycol
| CVS PAIN RELIEVING FIRST AID ANTISEPTIC
lidocaine hydrochloride, benzalkonium chloride spray |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - CVS Pharmacy (062312574) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.