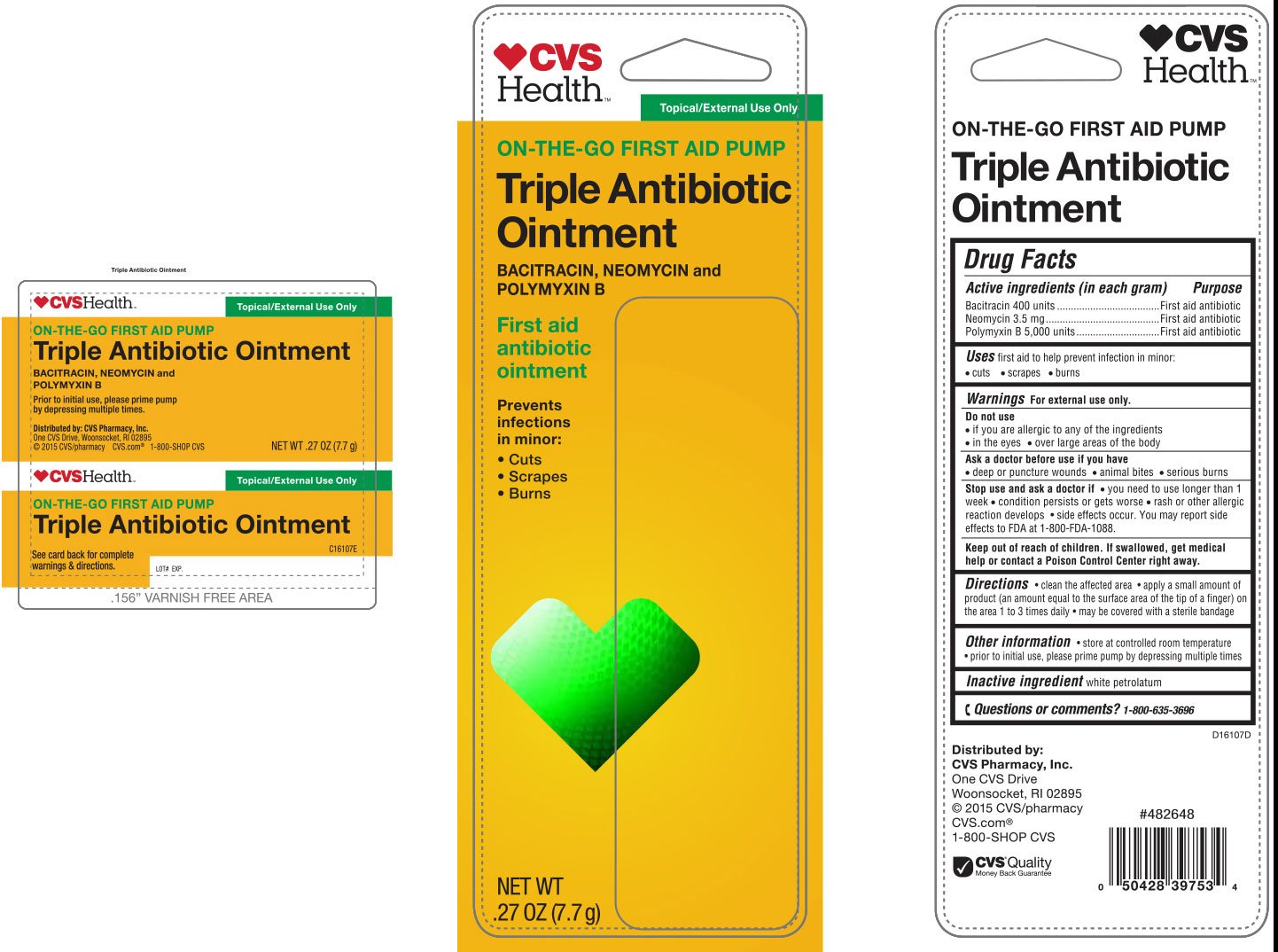
CVS Triple Antibiotic
Dosage form: ointment
Ingredients: BACITRACIN ZINC 400[USP'U] in 1g, NEOMYCIN SULFATE 3.5mg in 1g, POLYMYXIN B SULFATE 5000[USP'U] in 1g
Labeler: CVS Pharmacy
NDC code: 69842-610
Medically reviewed by Drugs.com. Last updated on Dec 25, 2023.
Active Ingredients (in each gram)
Bacitracin 400 units
Neomycin 3.5 mg
Polymyxin B 5,000 units
first aid to help prevent infection in minor:
• cuts • scrapes • burns
For external use only.
• if you are allergic to any of the ingredients
• in the eyes
• over large areas of the body
• deep or puncture wounds • animal bites • serious burns
• you need to use longer than 1 week
• condition persists or gets worse
• rash or other allergic reaction develops • side effects occur. You may report side effects to FDA at 1-800-FDA-1088.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
• clean the affected area • apply a small amount of product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily • may be covered with a sterile bandage
white petrolatum
First Aid Antibiotic
•store at controlled room temperature
• prior to initial use, please prime pump by depressing multiple times
1-800-635-3696
| CVS TRIPLE ANTIBIOTIC
bacitracin, neomycin, polymyxin b ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.