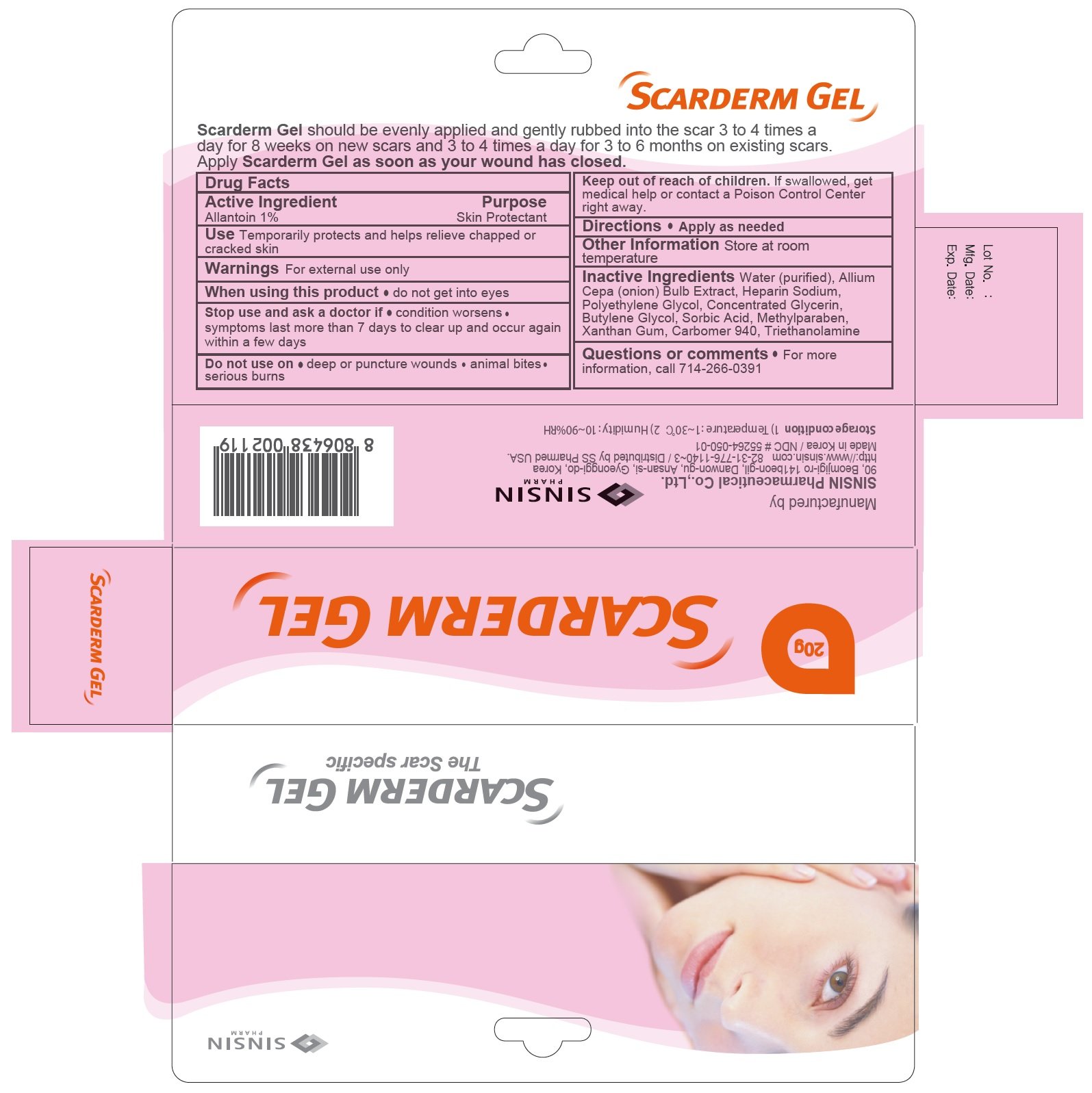
Scarderm
Dosage form: gel
Ingredients: Allantoin 10mg in 1g
Labeler: Sinsin Pharmaceutical Co., Ltd.
NDC code: 55264-050
Medically reviewed by Drugs.com. Last updated on May 22, 2023.
Drug Facts
Allantoin 1%
Skin Protectant
Temporatrily protects and helps relieve chapped or cracked skin
For external use only
When using this product do not get into eyes
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
- deep or puncture wounds
- animal bites
- serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Apply as needed.
Store at room temperature
Water (purified), Allium Cepa (Onion) Bulb Extract, Heparin Sodium, Polyethylene Glycol, Concentrated Glycerin, Butylene Glycol, Sorbic Acid, Methylparaben, Jantangeom, Carbomer 940, Triethanolamine
For more information call 714-266-0391
| SCARDERM
allantoin gel |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Sinsin Pharmaceutical Co., Ltd. (823149161) |
| Registrant - Sinsin Pharmaceutical Co., Ltd. (823149161) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.