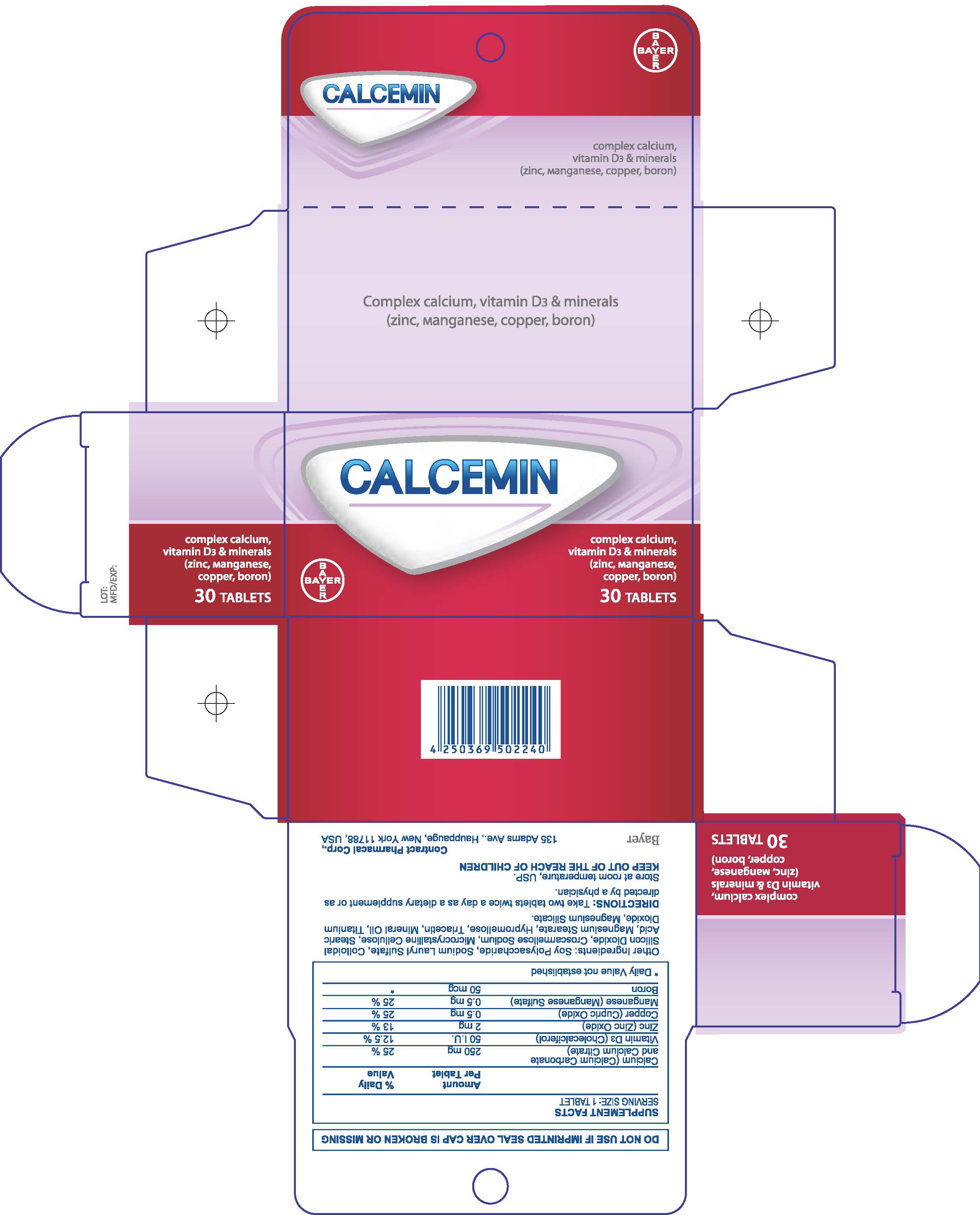
Calcemin
Dosage form: tablet, film coated
Ingredients: PREVITAMIN D3 0.75mg, ZINC 2mg, BORON 50ug
Labeler: Bayer HealthCare LLC.
NDC code: 0280-0271
Medically reviewed by Drugs.com. Last updated on Oct 25, 2023.
| CALCEMIN
calcium carbonate tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Contract Pharmacal | 968335112 | manufacture(0280-0271), pack(0280-0271) | |
Document Id: 9687dfa1-2ed0-a41d-e053-2995a90a60a0
Set id: 30ec5342-c010-1ad1-e054-00144ff8d46c
Version: 2
Effective Time: 20191104
Bayer HealthCare LLC.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.