BRONCOCHEM EXPECTORANT II
Dosage form: syrup
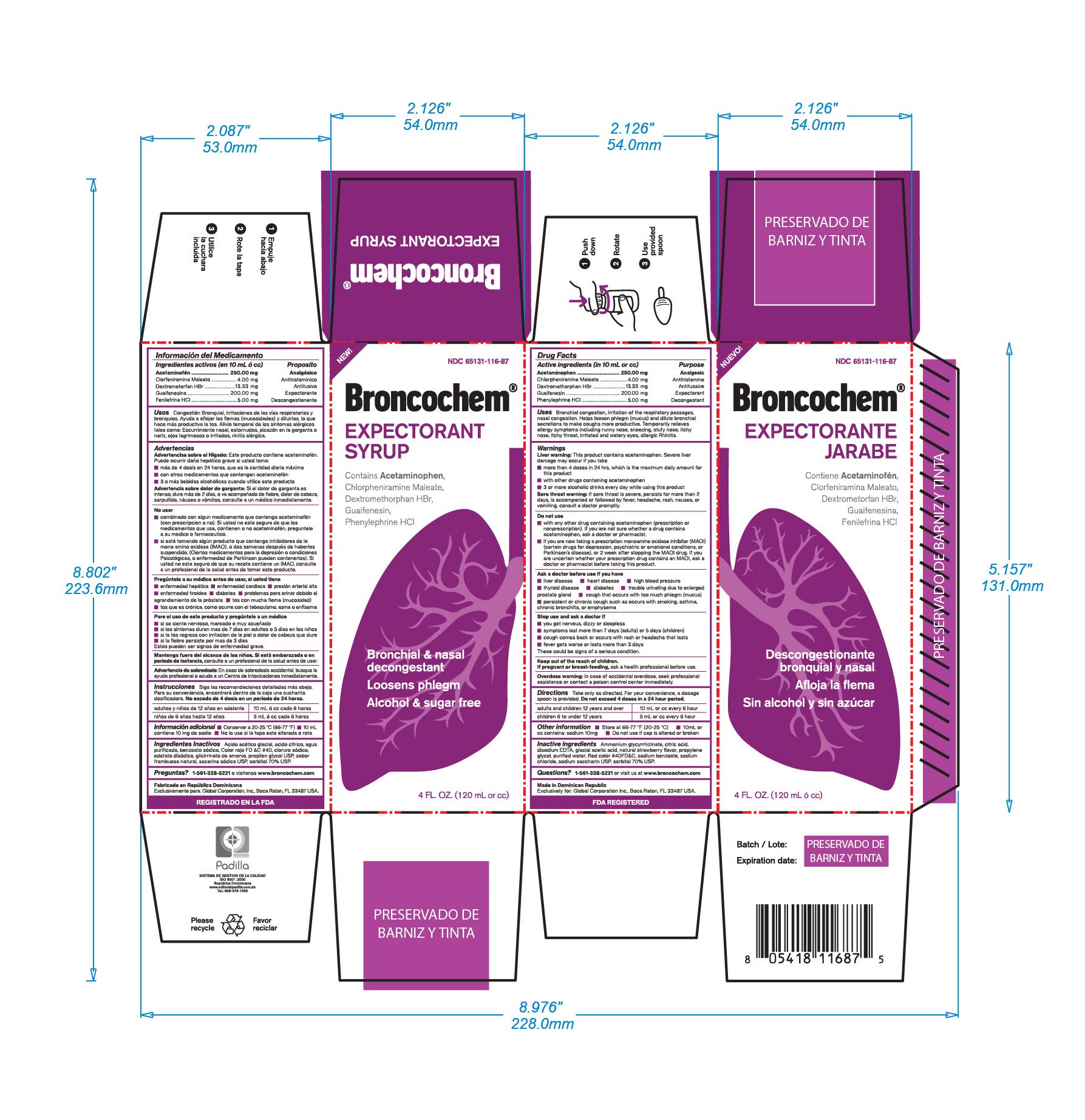
Ingredients: ACETAMINOPHEN 250mg in 10mL, CHLORPHENIRAMINE MALEATE 4mg in 10mL, DEXTROMETHORPHAN HYDROBROMIDE 13.33mg in 10mL, GUAIFENESIN 200mg in 10mL, PHENYLEPHRINE HYDROCHLORIDE 5mg in 10mL
Labeler: LABORATORIO MAGNACHEM INTERNATIONAL SRL
NDC code: 65131-116
Medically reviewed by Drugs.com. Last updated on Dec 11, 2023.
Unless directed by a physician do not take this product if persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, emphysema, or if cough is accompanied by excessive phlem (mucus). Likewise if you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland, if nervousness, dizziness, or sleeplesness occur, discontinue use or consult a doctor, if symptoms do not improve within 7 days or are accompainied by fever, consult a doctor. A persistent cough may be a sing of a serious condition. Stop use and ask a doctor if symptoms persist or last long that 5 days (children) or 7 days (adults), tend to return, arash, or persistent headache. As with any drug, if you are pregnant or nursing a baby; seek the advice of a healh professional before using this product. Unless directed by a doctor, do not take this product if you are presently taking another product containing Pseudoephedrine HCl, or if you are taking sedatives or trankilizers; it may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. Use caution when driving a motor vehicle or operating machinery.
Acetaminophen
Chlorpheniramine Maleate
Dextromethorphan HBr
Guaifenesin
Phenylephrine HCl
Analgesic
Antihistaminic
Antotussive
Expectorant
Decongestant
In case of accidental overdose, seek professional assistancve or contact a poison control center inmediately
Bronchial congestion
Irritation of the respiratory passages
Nasal congestion
Helps loosen phlegm (mucus) and dilute bronchial secretions to make coughs more productive
Antitussive
Temporarilly relieves allergy symptoms including runny nose, sneezing, stuffy nose, itchy nose, itchy throat, irritated and watery eyes, allergic rhinitis
Do not exceed 4 doses in a 24 hour period
Adults and chuildren 12 years and over: (10mL or cc) using metered dosage spoon every 6 hours.
Children 6 years up to 12 years: (5mL or cc) using metered dosage spoon every 6 hours
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI). (certain drugs for depression, psychiatric or emotional conditions, or Parkinson`s disease), or 2 weeks after stopping the MAOI drug. If you are uncertain whether your presciption drug contains an MAOI, consult a health professional before taking this product
Aloe Vera, Ammonium Glycyrrhizinate, Citric acid, Disodium EDTA, Glacial Acetic Acid, Natural Raspberry flavor, Propylene Glycol USP, Purified Water, FD&C Red No.40, Sodium Benzoate, Sodium Saccharin USP, Sorbitol 70%.
Aloe Vera, Ammonium Glycyrrhizinate, Citric acid, Disodium EDTA, Glacial Acetic Acid, Natural Raspberry flavor, Propylene Glycol USP, Purified Water, FD&C Red No.40, Sodium Benzoate, Sodium Saccharin USP, Sorbitol 70%.
| BRONCOCHEM
EXPECTORANT II
acetaminophen-chlorpheniramine maleate-dextromethorphan hbr-guaifenesin-phenylephrine hcl syrup |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) |
| Registrant - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LABORATORIO MAGNACHEM INTERNATIONAL SRL | 871446100 | manufacture(65131-116) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.