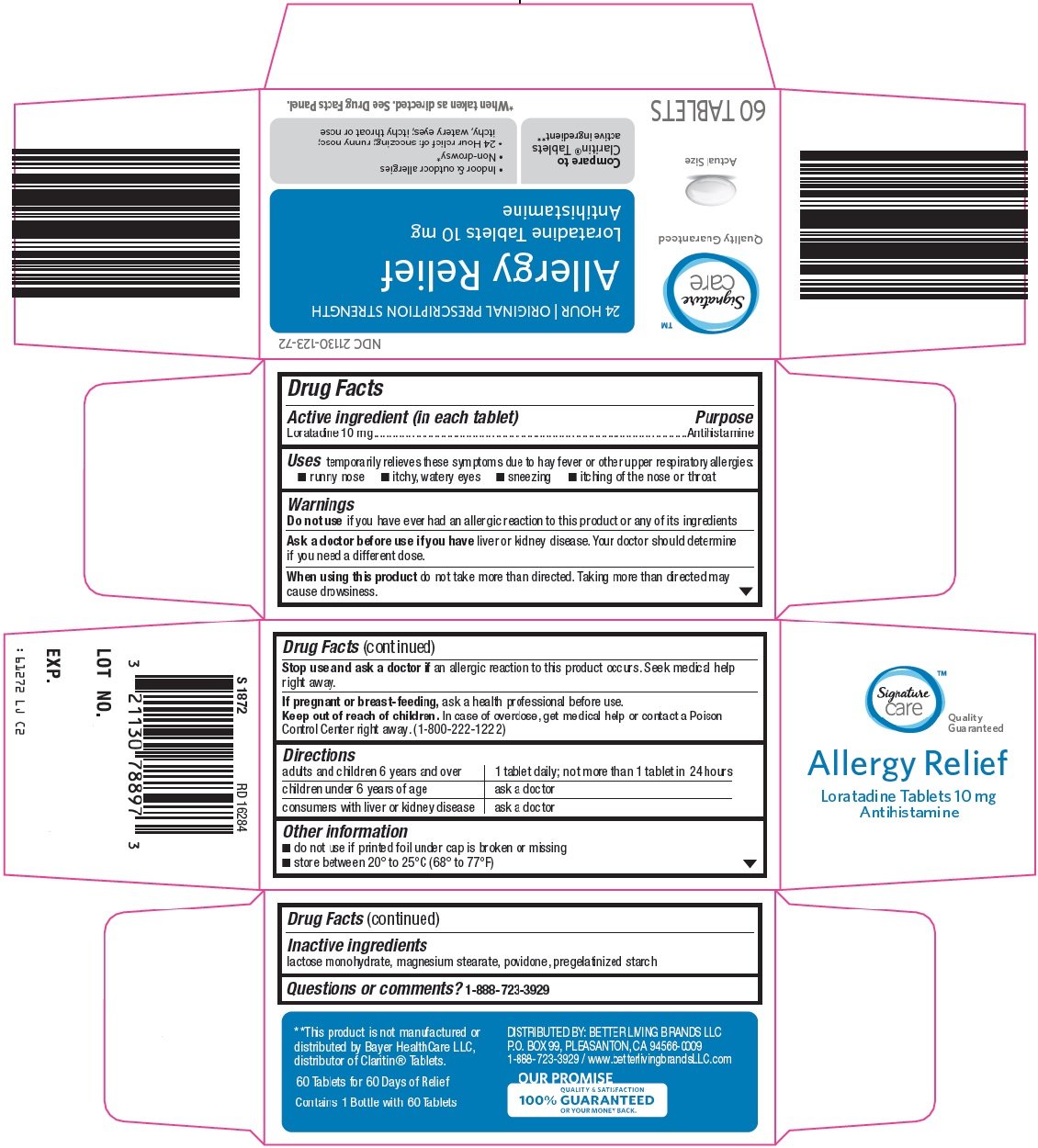
Signature Care Allergy Relief
Dosage form: tablet
Ingredients: LORATADINE 10mg
Labeler: Safeway
NDC code: 21130-123
Medically reviewed by Drugs.com. Last updated on Dec 6, 2023.
Loratadine 10 mg
Antihistamine
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
if you have ever had an allergic reaction to this product or any of its ingredients
liver or kidney disease. Your doctor should determine if you need a different dose.
do not take more than directed. Taking more than directed may cause drowsiness.
an allergic reaction to this product occurs. Seek medical help right away.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
|
adults and children 6 years and over |
1 tablet daily; not more than 1 tablet in 24 hours |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
- •
- do not use if printed foil under cap is broken or missing
- •
- store between 20° to 25°C (68° to 77°F)
lactose monohydrate, magnesium stearate, povidone, pregelatinized starch
1-888-723-3929
24 HOUR | ORIGINAL PRESCRIPTION STRENGTH
Allergy Relief
Loratadine Tablets 10 mg
Antihistamine
Actual Size
Compare to Claritin® Tablets active ingredient
Indoor & outdoor allergies
Non-drowsy*
24 Hour relief of: sneezing; runny nose; itchy, watery eyes; itchy throat or nose
60 TABLETS
*When taken as directed. See Drug Facts Panel.
| SIGNATURE CARE ALLERGY RELIEF
loratadine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Safeway (009137209) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.