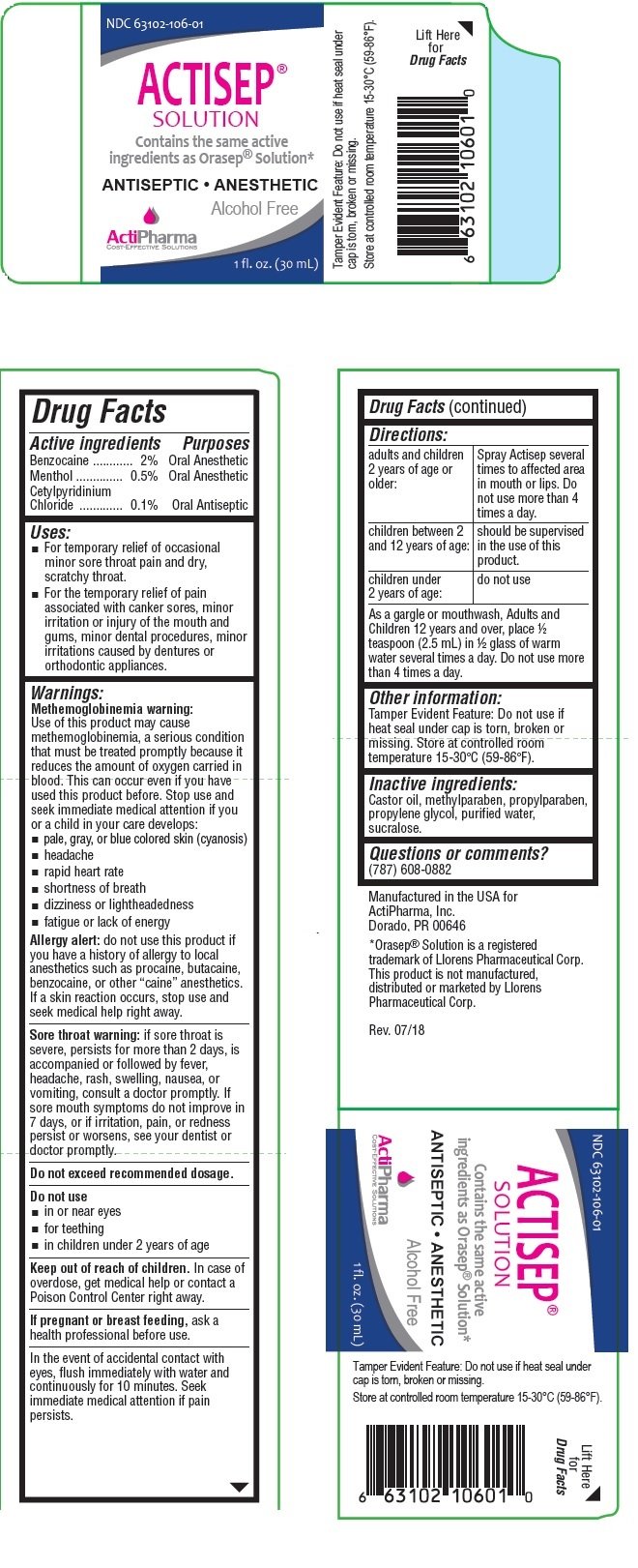
ACTISEP
Dosage form: solution
Ingredients: BENZOCAINE 2g in 100mL, MENTHOL, UNSPECIFIED FORM 0.5g in 100mL, CETYLPYRIDINIUM CHLORIDE 0.1g in 100mL
Labeler: Actipharma, Inc
NDC code: 63102-106
Medically reviewed by Drugs.com. Last updated on Nov 27, 2023.
Benzocaine .............................2%
Menthol ................................0.5%
Cetylpyridinium Chloride ........0.1%
Oral Anesthetic
Oral Anesthetic
Oral Antiseptic
• For temporary relief of occasional minor sore throat pain and dry, scratchy throat.
• For the temporary relief of pain associated with canker sores, minor irritation or injury of the mouth and gums, minor dental procedures, minor irritations caused by dentures or orthodontic appliances.
Methemoglobinemia warning:
Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other “caine” anesthetics. If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, or if irritation, pain, or redness persist or worsens, see your dentist or doctor promptly.
Do not exceed recommended dosage.
Do not use
- in or near eyes
- for teething
- in children under 2 years of age
If pregnant or breast feeding, ask a health professional before use.
In the event of accidental contact with eyes, flush immediately with water and continuously for 10 minutes. Seek immediate medical attention if pain persists.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
| adults and children 2 years of age or older: | Spray Actisep several times to affected area in mouth or lips. Do not use more than 4 times a day. |
| children between 2 and 12 years of age: | should be supervised in the use of this product. |
| children under 2 years of age: | do not use |
As a gargle or mouthwash, Adults and Children 12 years and over, place ½ teaspoon (2.5 mL) in ½ glass of warm
water several times a day. Do not use more than 4 times a day.
Castor oil, methylparaben, propylparaben, propylene glycol, purified water, sucralose.
Tamper Evident Feature: Do not use if heat seal under cap is torn, broken or missing. Store at controlled room temperature 15-30°C (59-86°F).
(787) 608-0882
Contains the same active ingredients as Orasep® Solution*
ANTISEPTIC • ANESTHETIC
Alcohol Free
Manufactured in the USA for
ActiPharma, Inc.
Dorado, PR 00646
*Orasep® Solution is a registered trademark of Llorens Pharmaceutical Corp.
This product is not manufactured, distributed or marketed by Llorens Pharmaceutical Corp.
| ACTISEP
benzocaine, menthol, cetylpyridinium chloride solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Actipharma, Inc (079340948) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.