Hello Kitty Hand Sanitizer
Dosage form: liquid
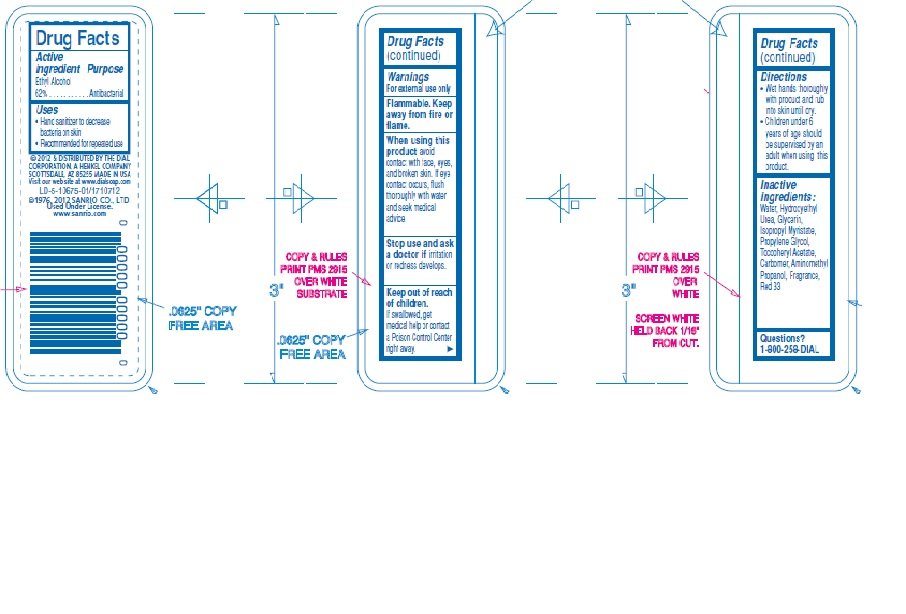
Ingredients: ALCOHOL 0.62mL in 1mL
Labeler: The Dial Corporation, a Henkel company
NDC code: 54340-256
Medically reviewed by Drugs.com. Last updated on Jul 24, 2023.
Ethyl alcohol 62%
Antibacterial
- Hand Sanitizer
- For external use only
- Flammable. Keep away from fire or flame.
avoid contact with face, eyes, and broken skin. If eye contact occurs, flush thoroughly with water and seek medical advice
Stop use and ask a doctor if irritation or redness develops.
If swallowed, get medical help or contact a Poison Control Center right away.
Wet hands thoroughly with product and rub into skin until dry
Children under 6 years of age should be supervised by an adult when using this product.
Water (Aqua), Hydroxyethyl Urea, Glycerin, Isopropyl Myristate, Propylene Glycol, Tocopheryl Acetate, Carbomer, Aminomethyl Propanol, Fragrance (parfume), Red 33 (CI 17200)
1-800-258-3425
Topical liquid
| HELLO KITTY HAND SANITIZER
hand sanitizer liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - The Dial Corporation, a Henkel company (070252531) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.