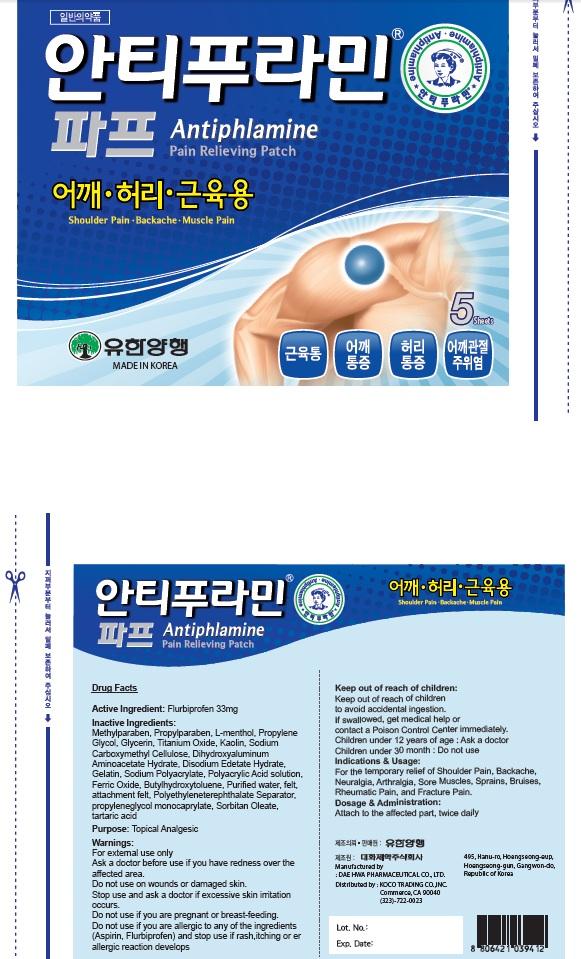
Antiphlamine Pain Relieving
Dosage form: patch
Ingredients: Flurbiprofen 33mg
Labeler: HANUL TRADING CO., LTD.
NDC code: 69642-1300
Medically reviewed by Drugs.com. Last updated on Oct 3, 2023.
Active Ingredient: Flurbiprofen 33mg
Inactive Ingredients: Methylparaben, Propylparaben, L-menthol, Propylene Glycol, Glycerin, Titanium Oxide, Kaolin, Sodium Carboxymethyl Cellulose, Dihydroxyaluminum Aminoacetate Hydrate, Disodium Edetate Hydrate, Gelatin, Sodium Polyacrylate, Polyacrylic Acid solution, Ferric Oxide, Butylhydroxytoluene, Purified water, felt, attachment felt, Polyethyleneterephthalate Separator, propyleneglycol monocaprylate, Sorbitan Oleate, tartaric acid
Purpose: Topical Analgesic
Warnings: For external use only Ask a doctor before use if you have redness over the affected area. Do not use: on wounds or damaged skin. Stop use and ask a doctor if excessive skin irritation occurs. Do not use if you are pregnant or breast feading. Do not use if you are allergic to any of the ingredients(Aspirin, Flurbiprofen) and stop use if rash, itching or allergic reaction develops.
Keep out of reach of children: Keep out of reach of children to avoid accidental ingestion. If swallowed, get medical help or contact a Poison Control Center immediately. Children under 12 years of age: Ask a doctor Children under 30 month: Do not use
Indications & Usage: For the temporary relief of Shoulder Pain, Backache, Neuralgia, Arthralgia, Sore Muscles, Sprains, Bruises, Rheumatic Pain, and Fracture Pain.
Dosage & Administration: Attach to the affected part, twice daily
| ANTIPHLAMINE PAIN RELIEVING
flurbiprofen patch |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - HANUL TRADING CO., LTD. (689512982) |
| Registrant - HANUL TRADING CO., LTD. (689512982) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| HANUL TRADING CO., LTD. | 689512982 | repack(69642-1300) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Dae Hwa Pharm Co., Ltd. | 688004324 | manufacture(69642-1300) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| KOCO TRADING CO., INC. | 079457993 | wholesale drug distributor(69642-1300) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.