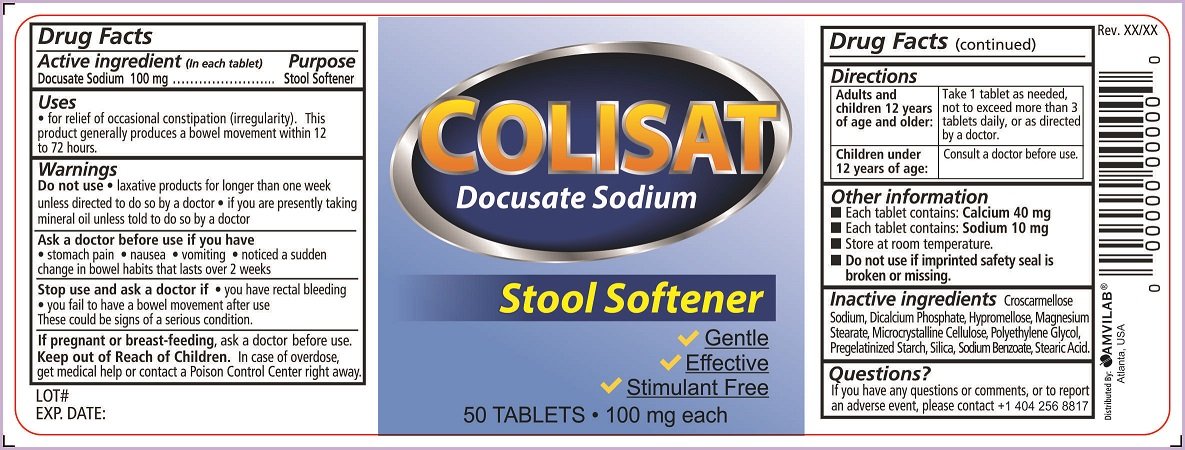
Colistat
Dosage form: tablet, film coated
Ingredients: DOCUSATE SODIUM 100mg
Labeler: Amvilab LLC
NDC code: 69975-750
Medically reviewed by Drugs.com. Last updated on Jul 6, 2023.
Docusate Sodium 100 mg
Stool Softener
- for relief of occasional constipation (irregularity).This product generally produces a bowel movement within 12 to 72 hours.
- laxative products for longer than one week unless directed to do so by a doctor
- if you are presently taking mineral oil unless told to do so by a doctor
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- you have rectal bleeding
- you fail to have a bowel movement after use
These could be signs of a serious condition.
ask a doctor before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
| Adults and children 12 years of age and older: | Take 1 tablet as needed, not to exceed more than 3 tablets daily, or as directed by a doctor. |
| Children under 12 years of age: | Consult a doctor before use. |
- Each tablet contains: Calcium 40 mg
- Each tablet contains: Sodium 10 mg
- Store at room temperature.
- Do not use if imprinted safety seal is broken or missing
Croscarmellose Sodium, Dicalcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Pregelatinized Starch, Silica, Sodium Benzoate, Stearic Acid.
If you have any questions or comments, or to report an adverse event, please contact +1 404 256 8817
| COLISTAT
docusate sodium tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Amvilab LLC (006092439) |
| Registrant - Gemini Pharmaceuticals, Inc. dba Plus Pharma (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Gemini Pharmaceuticals, Inc. dba Plus Pharma | 055942270 | manufacture(69975-750) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.