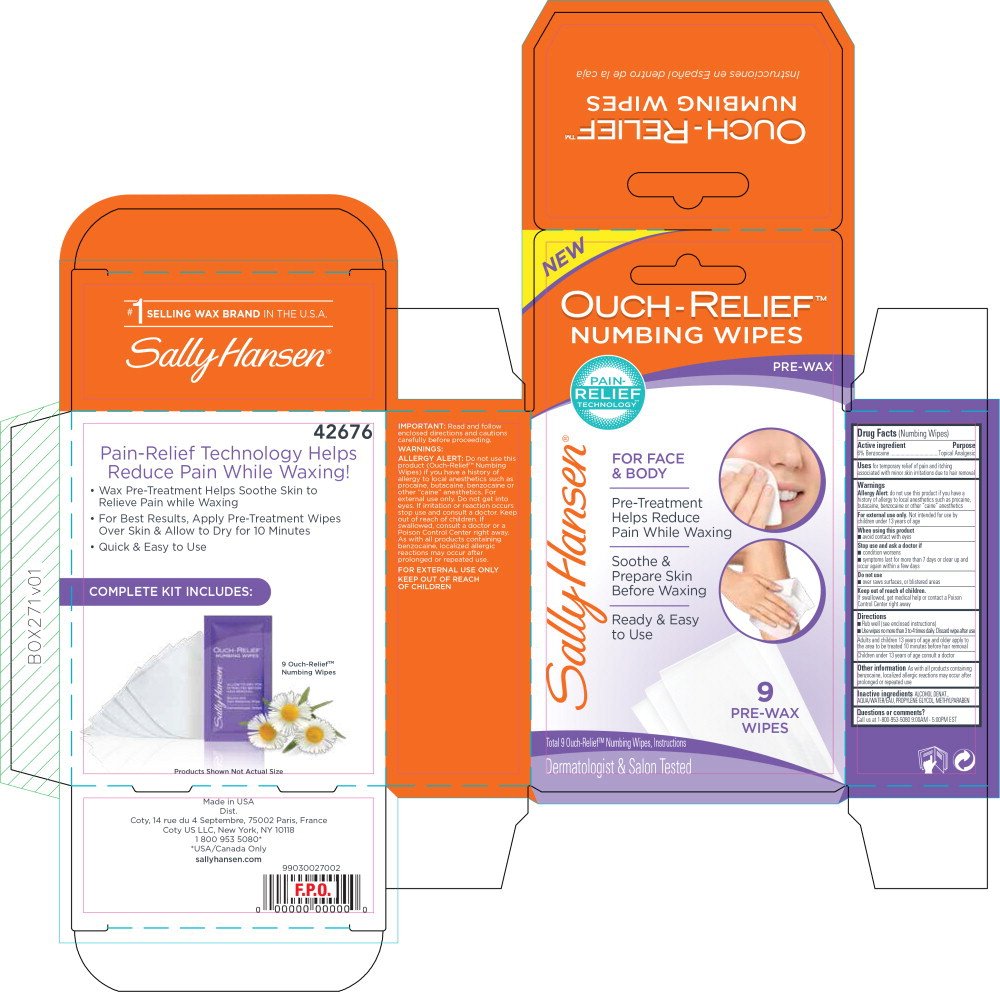
Sally Hansen Ouch-Relief Numbing Wipes
Dosage form: cloth
Ingredients: BENZOCAINE 0.0543g
Labeler: Coty US LLC
NDC code: 66184-156
Medically reviewed by Drugs.com. Last updated on Feb 1, 2024.
Drug Facts (Numbing Wipes)
6% Benzocaine
Topical Analgesic
for temporary relief of pain and itching associated with minor skin irritations due to hair removal
Allergy Alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
For external use only. Not intended for use by children under 13 years of age
- avoid contact with eyes
- condition worsens
- symptoms last for more than 7 days or clear up and occur again within a few days
- over raws surfaces, or blistered areas
If swallowed, get medical help or contact a Poison Control Center right away
- Rub well (see enclosed instructions)
- Use wipes no more than 3 to 4 times daily. Discard wipe after use
Adults and children 13 years of age and older apply to :he area to be treated 10 minutes before hair removal Children under 13 years of age consult a doctor
As with all products containing benzocaine, localized allergic reactions may occur after prolonged or repeated use
ALCOHOL DENAT., AQUA/WATER/EAU, PROPYLENE GLYCOL, METHYLPARABEN
Call us at 1-800-953-5080 9:00AM - 5:00PM EST
OUCH-RELIEF™
NUMBING WIPES
PRE-WAX
PAIN-
RELIEF
TECHNOLOGY
FOR FACE
& BODY
Pre-Treatment
Help Reduce
Pain While Waxing
Soothe &
Prepare Skin
Before Waxing
Ready & Easy
to Use
9
PRE-WAX
WIPES
Total 9 Ouch-Relief™ Numbing Wipes, Instructions
Dermatologist & Salon Tested
| SALLY HANSEN OUCH-RELIEF NUMBING WIPES
benzocaine cloth |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - Coty US LLC (039056361) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.