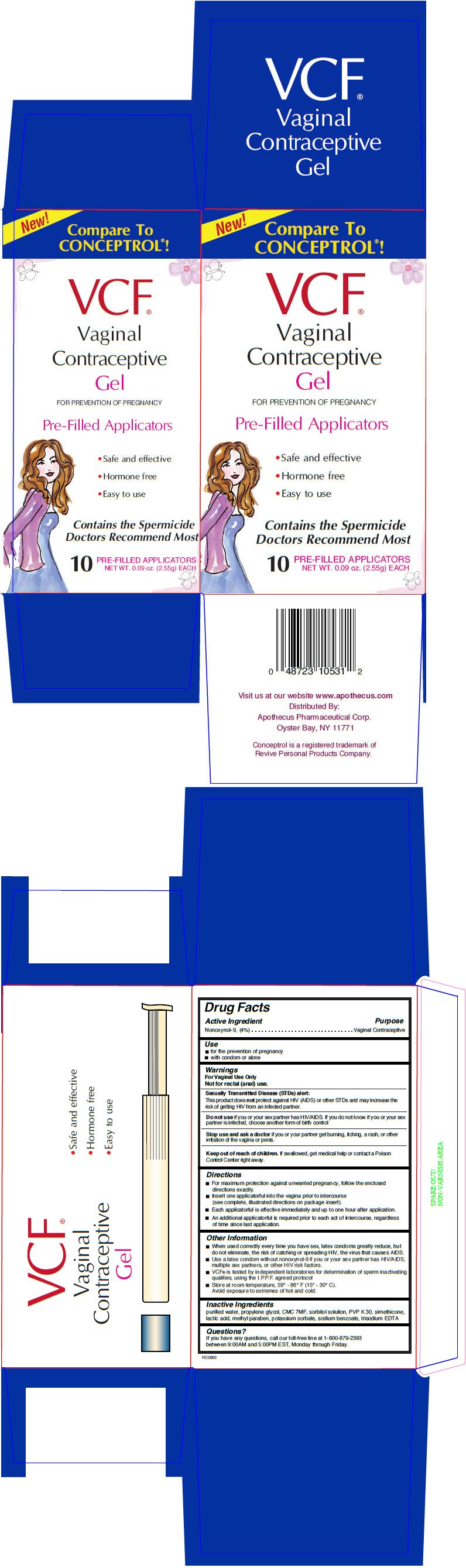
VCF CONTRACEPTIVE PRE-FILLED APPLICATORS
Dosage form: gel, metered
Ingredients: NONOXYNOL-9 4g in 100g
Labeler: APOTHECUS PHARMACEUTICAL CORP
NDC code: 52925-512
Medically reviewed by Drugs.com. Last updated on Dec 1, 2023.
Drug Facts
Nonoxynol-9, (4%)
Vaginal Contraceptive
- for the prevention of pregnancy
- with condom or alone
For Vaginal Use Only
Not for rectal (anal) use.
This product does not protect against HIV (AIDS) or other STDs and may increase the risk of getting HIV from an infected partner.
Do not use if you or your sex partner has HIV/AIDS. If you do not know if you or your sex partner is infected, choose another form of birth control
Stop use and ask a doctor if you or your partner get burning, itching, a rash, or other irritation of the vagina or penis.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- For maximum protection against unwanted pregnancy, follow the enclosed directions exactly.
- Insert one applicatorful into the vagina prior to intercourse (see complete, illustrated directions on package insert).
- Each applicatorful is effective immediately and up to one hour after application.
- An additional applicatorful is required prior to each act of intercourse, regardless of time since last application.
- When used correctly every time you have sex, latex condoms greatly reduce, but do not eliminate, the risk of catching or spreading HIV, the virus that causes AIDS.
- Use a latex condom without nonoxynol-9 if you or your sex partner has HIV/AIDS, multiple sex partners, or other HIV risk factors.
- VCF® is tested by independent laboratories for determination of sperm inactivating qualities, using the I.P.P.F. agreed protocol
- Store at room temperature, 59° - 86° F (15° - 30° C).
Avoid exposure to extremes of hot and cold.
purified water, propylene glycol, CMC 7MF, sorbitol solution, PVP K 30, simethicone, lactic acid, methyl paraben, potassium sorbate, sodium benzoate, trisodium EDTA
If you have any questions, call our toll-free line at 1-800-879-2393 between 9:00AM and 5:00PM EST, Monday through Friday.
Distributed By:
Apothecus Pharmaceutical Corp.
Oyster Bay, NY 11771
New!
Compare To
CONCEPTROL®!
VCF®
Vaginal
Contraceptive
Gel
FOR PREVENTION OF PREGNANCY
Pre-Filled Applicators
- Safe and effective
- Hormone free
- Easy to use
Contains the Spermicide
Doctors Recommend Most
10
PRE-FILLED APPLICATORS
NET WT. 0.09 oz. (2.55g) EACH
| VCF CONTRACEPTIVE PRE-FILLED APPLICATORS
nonoxynol-9 gel, metered |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - APOTHECUS PHARMACEUTICAL CORP (119263747) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.