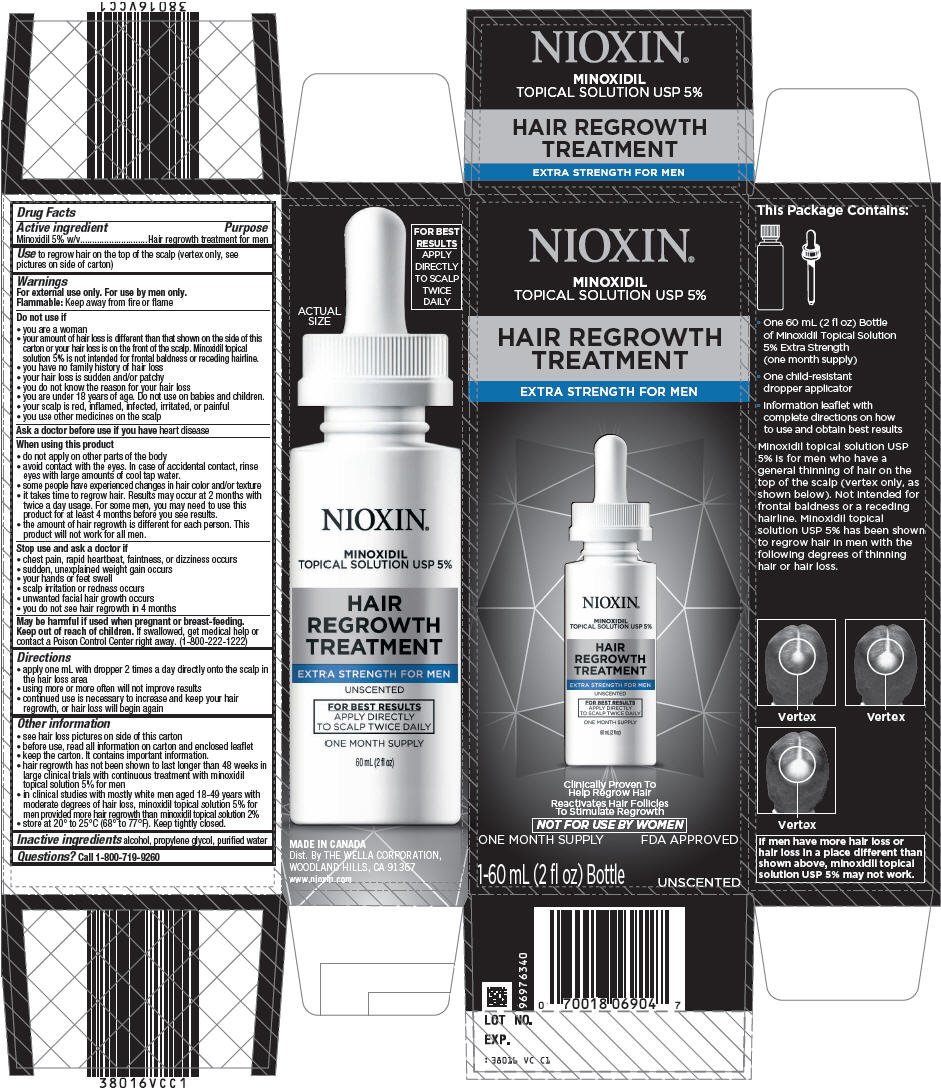
Nioxin Hair Regrowth Treatment Extra Strength for Men
Dosage form: solution
Ingredients: Minoxidil 5g in 100mL
Labeler: The Wella Corporation
NDC code: 69282-002
Medically reviewed by Drugs.com. Last updated on Oct 26, 2023.
Drug Facts
Minoxidil 5% w/v
Hair regrowth treatment for men
to regrow hair on the top of the scalp (vertex only, see pictures on side of carton)
For external use only. For use by men only.
Flammable: Keep away from fire or flame
- you are a woman
- your amount of hair loss is different than that shown on the side of this carton or your hair loss is on the front of the scalp. Minoxidil topical solution 5% is not intended for frontal baldness or receding hairline.
- you have no family history of hair loss
- your hair loss is sudden and/or patchy
- you do not know the reason for your hair loss
- you are under 18 years of age. Do not use on babies and children.
- your scalp is red, inflamed, infected, irritated, or painful
- you use other medicines on the scalp
Ask a doctor before use if you have heart disease
- do not apply on other parts of the body
- avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
- some people have experienced changes in hair color and/or texture
- it takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some men, you may need to use this product for at least 4 months before you see results.
- the amount of hair regrowth is different for each person. This product will not work for all men.
- chest pain, rapid heartbeat, faintness, or dizziness occurs
- sudden, unexplained weight gain occurs
- your hands or feet swell
- scalp irritation or redness occurs
- unwanted facial hair growth occurs
- you do not see hair regrowth in 4 months
May be harmful if used when pregnant or breast-feeding.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
- apply one mL with dropper 2 times a day directly onto the scalp in the hair loss area
- using more or more often will not improve results
- continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again
- see hair loss pictures on side of this carton
- before use, read all information on carton and enclosed leaflet
- keep the carton. It contains important information.
- hair regrowth has not been shown to last longer than 48 weeks in large clinical trials with continuous treatment with minoxidil topical solution 5% for men
- in clinical studies with mostly white men aged 18-49 years with moderate degrees of hair loss, minoxidil topical solution 5% for men provided more hair regrowth than minoxidil topical solution 2%
- store at 20° to 25°C (68° to 77°F). Keep tightly closed.
alcohol, propylene glycol, purified water
Call 1-800-719-9260
Dist. By THE WELLA CORPORATION,
WOODLAND HILLS, CA 91367
NIOXIN®
MINOXIDIL
TOPICAL SOLUTION USP 5%
HAIR REGROWTH
TREATMENT
EXTRA STRENGTH FOR MEN
Clinically Proven To
Help Regrow Hair
Reactivates Hair Follicles
To Stimulate Regrowth
NOT FOR USE BY WOMEN
ONE MONTH SUPPLY
FDA APPROVED
1-60 mL (2 fl oz) Bottle
UNSCENTED
| NIOXIN HAIR REGROWTH TREATMENT EXTRA STRENGTH FOR MEN
minoxidil solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - The Wella Corporation (829413157) |
| Registrant - Procter & Gamble Manufacturing Co. (004238200) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.