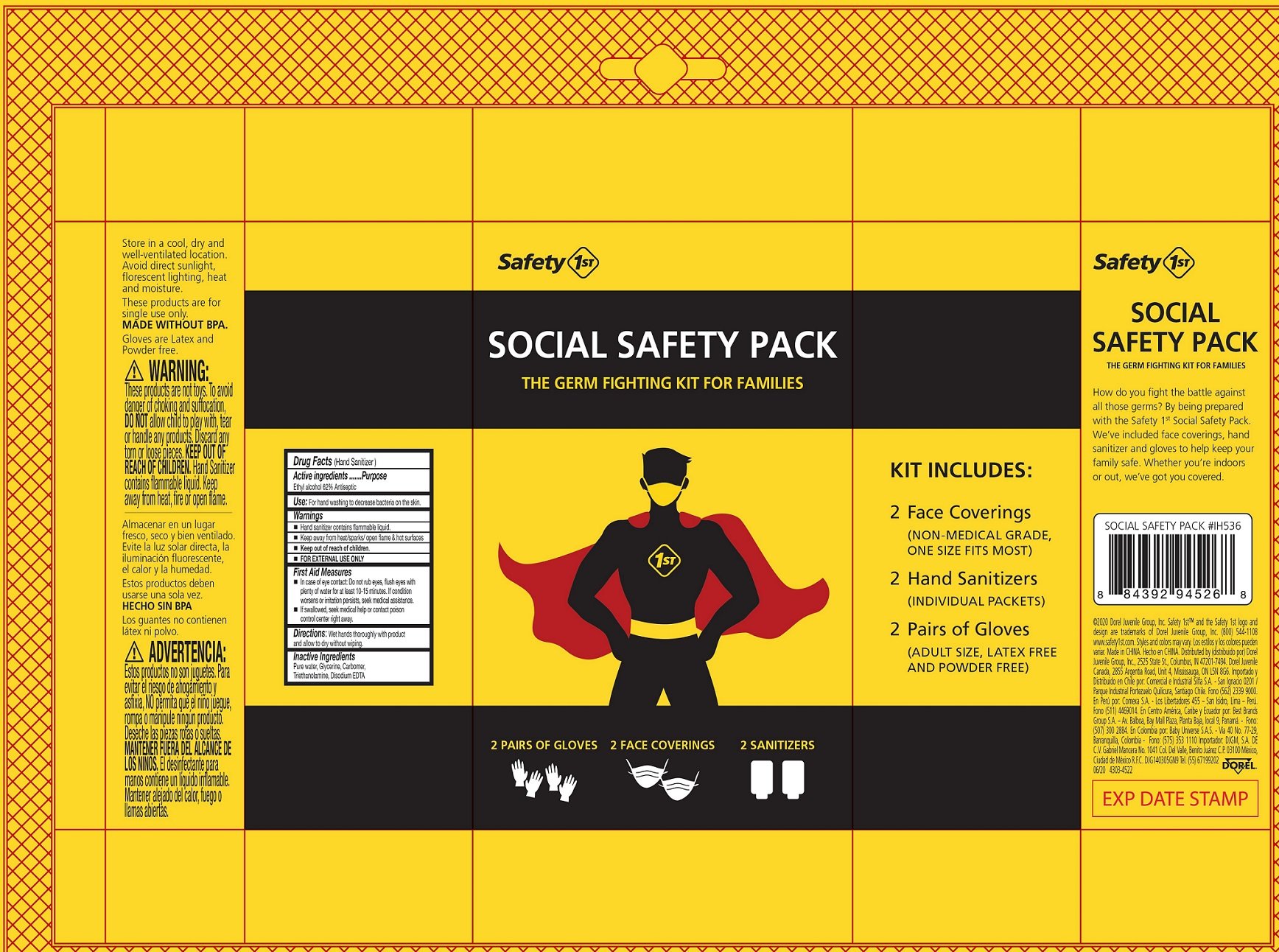
Safety 1st Social Safety Pack
Dosage form: kit
Ingredients: ALCOHOL 0.62mL in 1mL
Labeler: Dorel Juvenile Inc.
NDC code: 50428-0031
Medically reviewed by Drugs.com. Last updated on Aug 14, 2023.
Drug Facts
Active ingredients
Ethyl alcohol 62%
Purpose
Antiseptic
Use:
For hand washing to decrease bacteria on the skin.
Warnings
- Hand sanitizer contains flammable liquid.
- Keep away from heat/sparks/ open flame & hot surfaces
- FOR EXTERNAL USE ONLY
Keep out of reach of children.
First Aid Measures
- In case of eye contact: Do not rub eyes, flush eyes with plenty of water for at least 10-15 minutes. If condition worsens or irritation persists, seek medical assistance.
- If swallowed, seek medical help or contact poison control center right away.
Directions:
Wet hands thoroughly with product and allow to dry without wiping.
Inactive Ingredients
Pure water, Glycerine, Carbomer, Triethanolamine, Disodium EDTA
| SAFETY 1ST SOCIAL SAFETY PACK
alcohol kit |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - Dorel Juvenile Inc. (118841501) |
Document Id: ada35c48-806e-c3e0-e053-2995a90a3556
Set id: ada382f5-5925-37ce-e053-2a95a90af65d
Version: 1
Effective Time: 20200824
Dorel Juvenile Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.