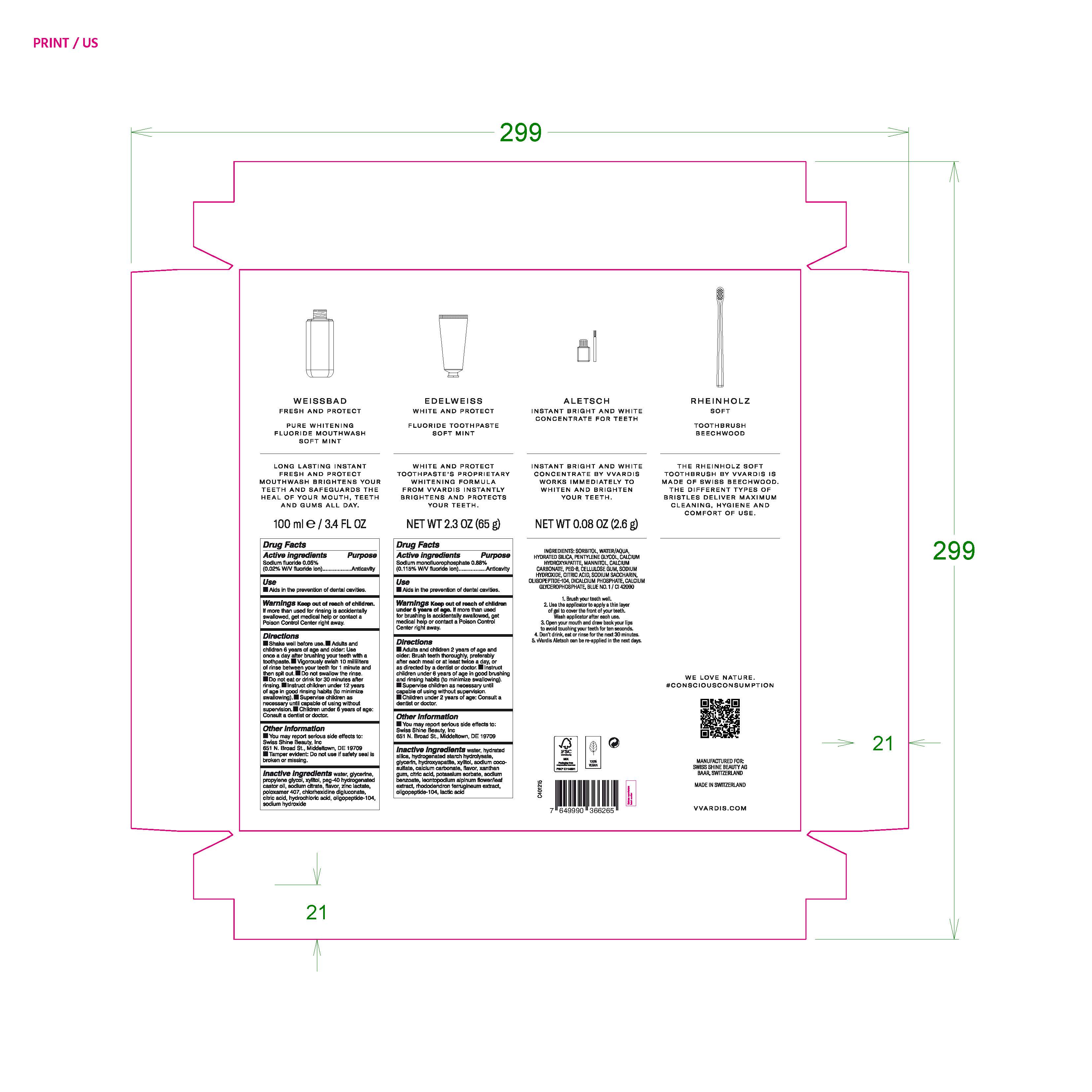
Active Ingredients
Sodium fluoride 0.05% (0.02% W/V fluoride ion)
Purpose Anticavity
Active Ingredients
Sodium monofluorophosphate 0.88% (0.115% W/V flouride ion)
Purpose Anticavity
Use
Aids in the prevention of dental cavities.
Warnings Keep out of reach of children. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Warnings Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Supervise children as necessary until capable of using without supervison.
Children under 2 years of age: Consult a dentist or doctor.
Supervise children as necessary until capable of using without supervision.
Children under 6 years of age: Consult a dentist or doctor.
Warnings Keep out of reach of children. If more than used for rinsing is accidently swallowed, get medical help or contact a Poison Control Center right away.
Warnings Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Shake well before use.
- Adults and children 6 years of age and older: Use once a day after brushing your teeth with a toothpaste.
- Vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit out.
- Do not swallow the rinse.
- Do not eat or drink for 30 minutes after rinsing.
- Instruct children under 12 years of age in good rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using wihtout supervision.
- Children under 6 years of age: Consult a dentist or doctor.
Directions
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing)
- Supervise children as necessary until capable of using without supervison.
- Children under 2 years of age: consult a dentist or doctor.
Inactive Ingredients
water, glycerine, propylene glycol, xylitol, peg-40 hydrogenated castor oil, sodium citrate, flavor, zinc lactate, poloxamer 407, chlorhexidine digluconate, citric acid, hydrochloric acid, oligopeptide-104, sodium hydroxide
Inactive Ingredients
water, hydrated silica, hydrogenated starch hydrolysate, glycerin, hydroxyapatite, xylitol, sodium coco-sulfate, calcium carbonate, flavor, xanthan gum, citric acid, potassium sorbate, sodium benzoate, leontopodium alpinum flower/leaf extract, rhododendron ferrugineum extract, oligopeptide-104, lactic acid.
Other information
You may report serious side effects to: Swiss Shine Beauty, Inc
651 N. Broad St., Middletown, DE 19709
Tamper evident: Do not use if safety seal is broken or missing.
WEISSBAD
FRESH AND PROTECT
PURE WHITENING
FLUORIDE MOUTHWASH
SOFT MINT
100 ml e /3.4 FL OZ
EDELWEISS
WHITE AND PROTECT
FLUORIDE TOOTHPASTE
SOFT MINT
NET WT 2.3 OZ (65 g)
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


