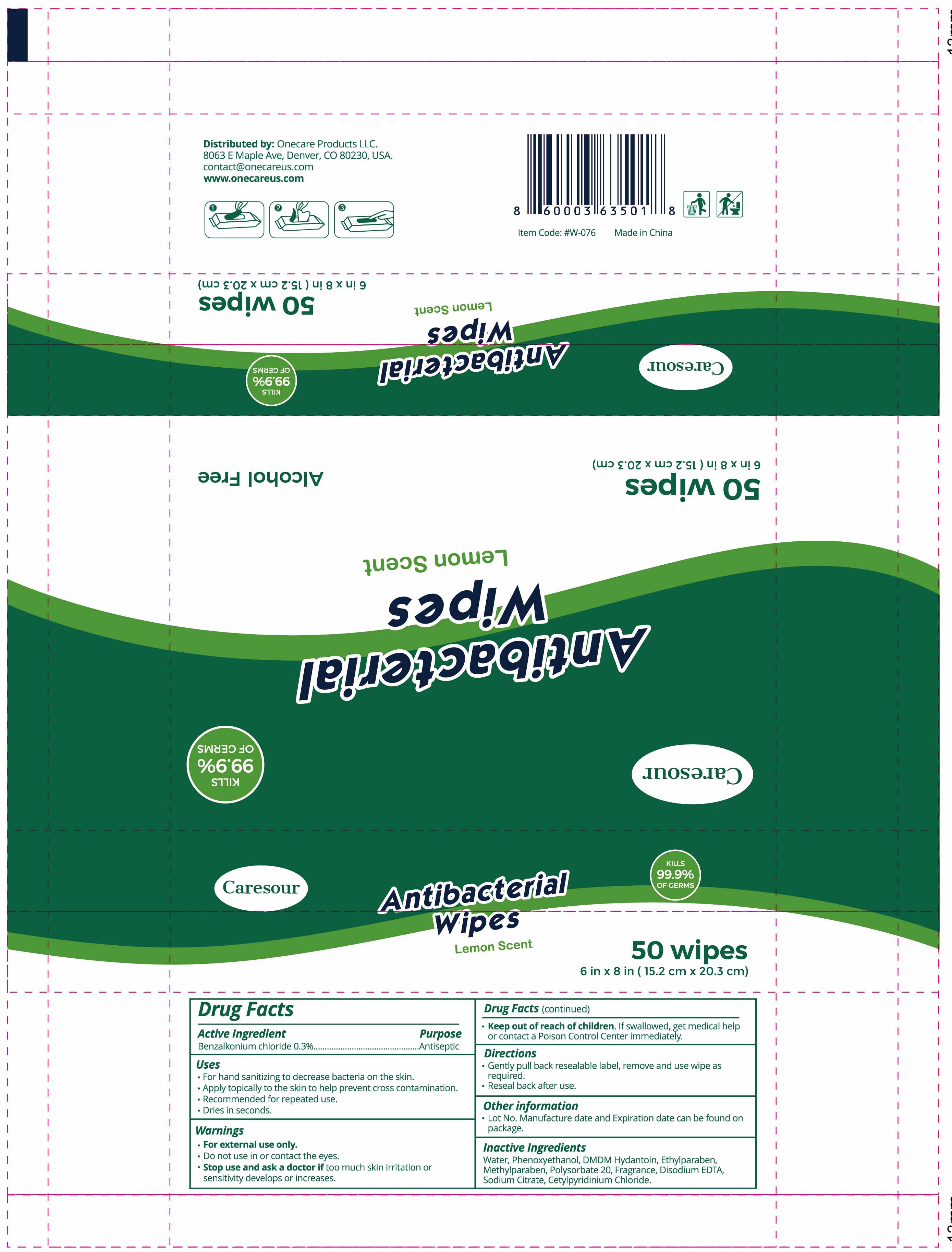
Caresour Antibacterial Wipes Lemon Scented
Dosage form: solution
Ingredients: BENZALKONIUM CHLORIDE 9.6mg
Labeler: Onecare Products LLC
NDC code: 75513-002
Medically reviewed by Drugs.com. Last updated on Jun 5, 2023.
Benzalkonium Chloride 0.3%
Antiseptic
For hand sanitizing to decrease bacteria on the skin.
Apply topically to the skin to help prevent cross contamination.
Recommended for repeated use.
Dries in seconds.
●For external use only.
Do not use in or contact the eyes.
●Stop use and ask a doctor if too much skin irritation or sensitivity develops or increases
●. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center
immediately.
●Gentally pull back reusablelabel, remove and use wipe as required.
●Reseal back after use
Lot No, Manufacture date, and Expiration date can be found on package
Water, Phenoxyethanol, DMDM Hydantoin, Ethylparaben, Methylparaben, Polysorbate 20, Fragrance, Disodium EDTA, Sodium Citrate, Cetylpyridinium Chloride
| CARESOUR ANTIBACTERIAL WIPES LEMON SCENTED
benzalkonium chloride solution |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Onecare Products LLC (129515451) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Kangna (Zhejiang) Medical Supplies Co., Ltd. | 554530173 | manufacture(75513-002) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.