Covaguard Mask
Dosage form: spray
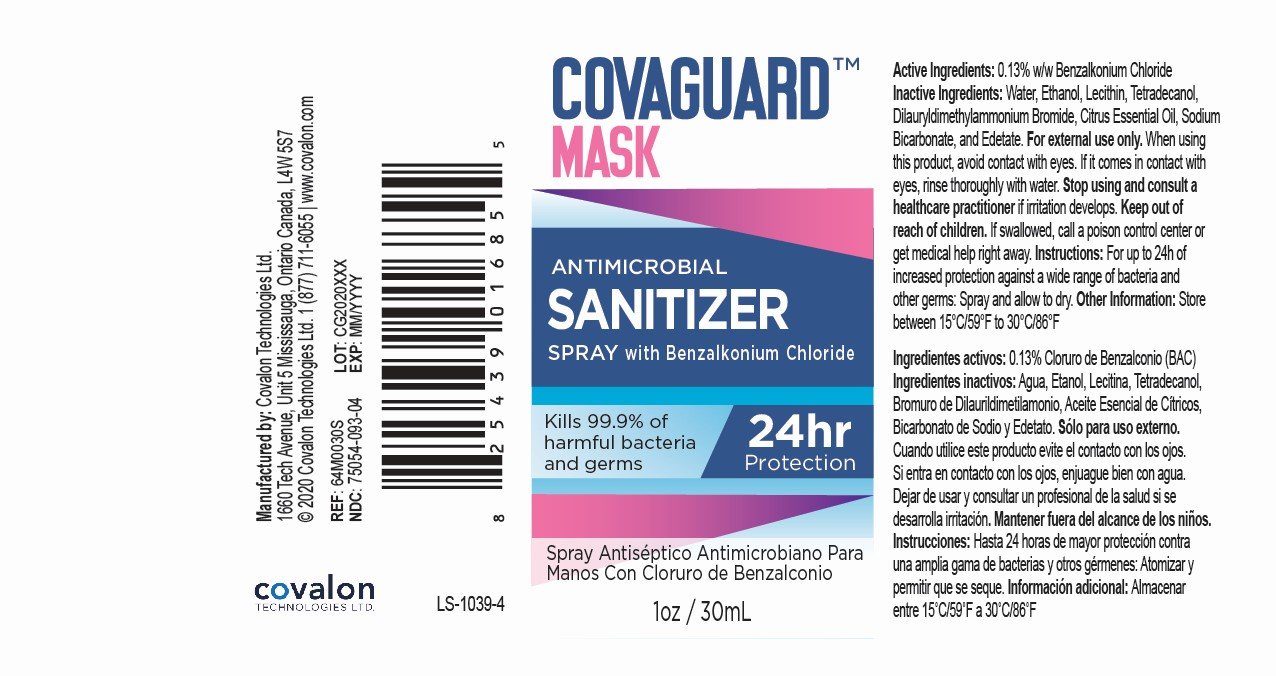
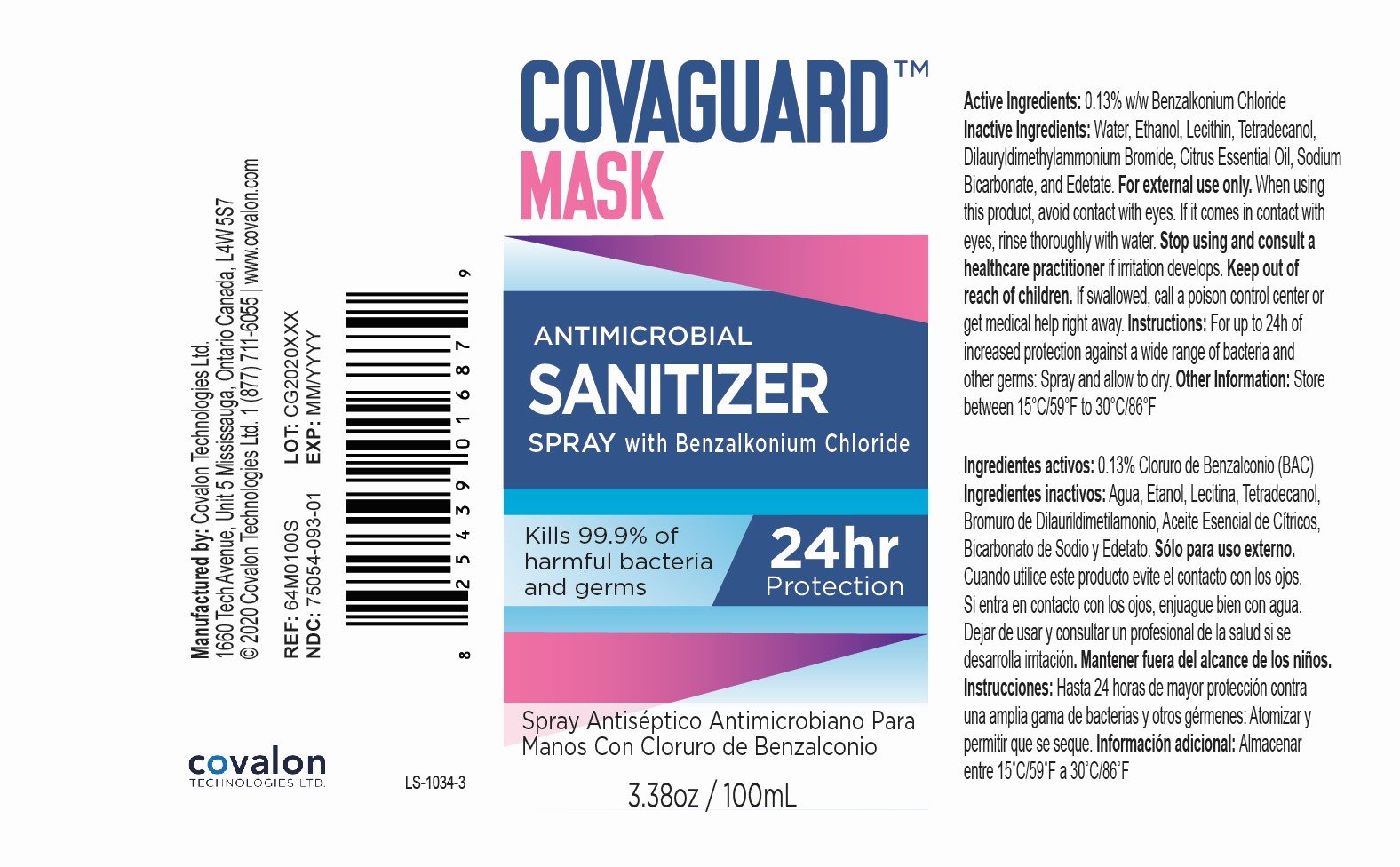
Ingredients: BENZALKONIUM CHLORIDE 0.13g in 100mL
Labeler: Covalon Technologies Ltd.
NDC code: 75054-093
Medically reviewed by Drugs.com. Last updated on Aug 10, 2023.
Benzalkonium Chloride 0.13% w/w
Antimicrobial Sanitizer
For external use only.
When using this product, avoid contact with eyes. If it comes in contact with eyes, rinse thoroughly with water.
Stop using and consult a healthcare practitioner if irritation develops.
Keep out of reach of children.
If swallowed, call a poison control center or get medical help right away.
Keep out of reach of children.
For up to 24h of increased protection against a wide range of bacteria and other germs: Spray and allow to dry.
Store between 15°C/59°F to 30°C/86°F.
Water, Ethanol, Soy Phosphatidylcholine, Myristyl Alcohol, Didodecyldimethylammonium Bromide, Orange Essential Oil, Sodium Bicarbonate, Disodium EDTA
1-877-711-6055
Spray liberally and allow to dry.
Antimicrobial Sanitizer.
| COVAGUARD MASK
antimicrobial sanitizer with benzalkonium chloride spray |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Covalon Technologies Ltd. (206936101) |
| Registrant - Covalon Technologies Ltd. (206936101) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Covalon Technologies Ltd. | 206936101 | manufacture(75054-093) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.