COCONUT LIME SCENTED HAND SANITIZER
Dosage form: gel
Ingredients: ALCOHOL 62mL in 100mL
Labeler: Greenbrier International, Inc.
NDC code: 33992-8962
Medically reviewed by Drugs.com. Last updated on May 19, 2023.
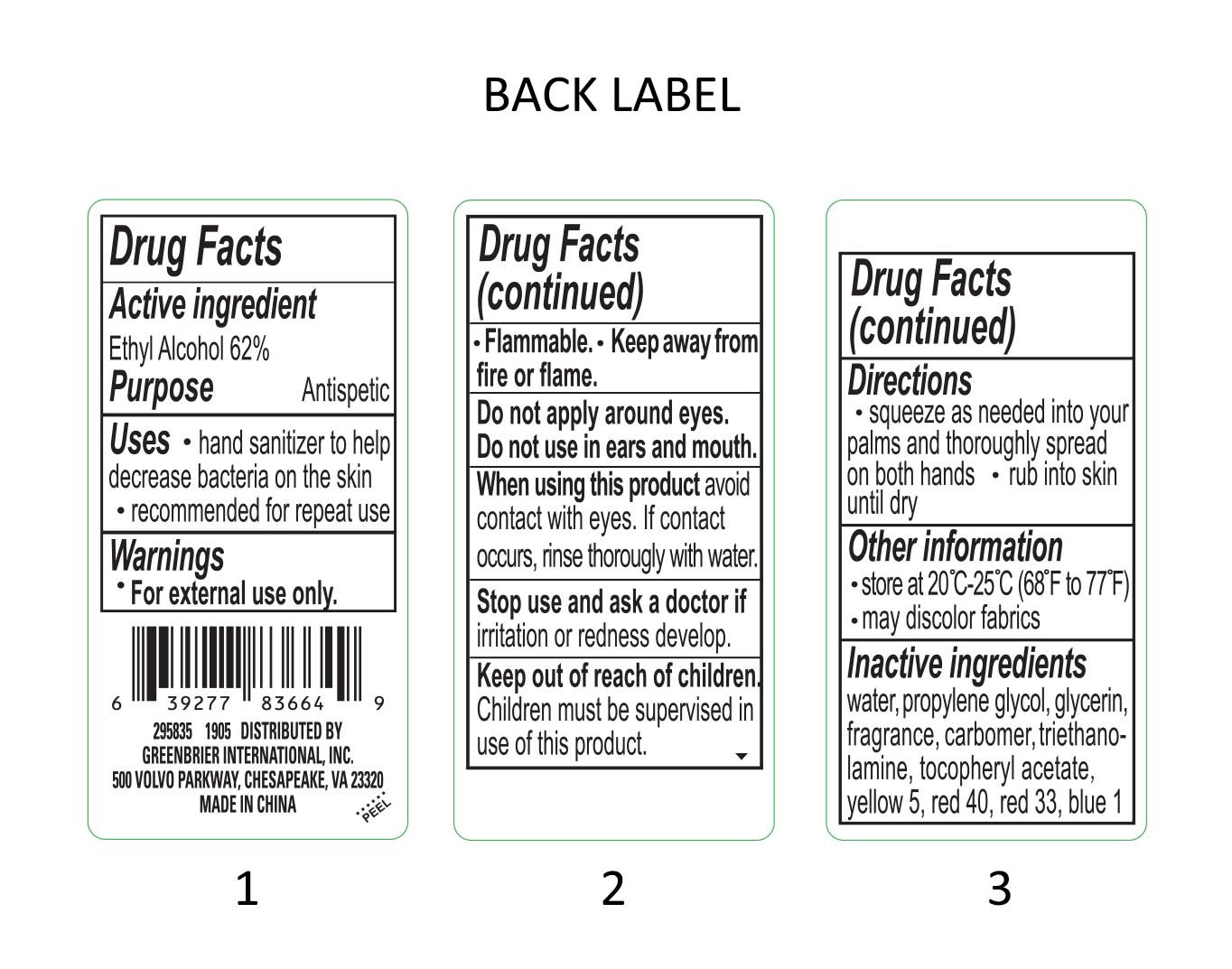
Drug Facts
Active Ingredient
Ethyl Alcohol 62%
Purpose
Antiseptic
Uses
- hand sanitizer to help decrease bacteria on the skin
- recommended for repeat use
Warnings
For external use only.
Flammable.
Keep away from fire or flame.
Do not apply around eyes.
Do not use in ears and mouth
When using this product avoid contact with eyes. If contact occurs, rinse thorougly with water.
Stop use and ask a doctor if irritation or redness develop.
Keep out of reach of children. Children must be supervised in use of this product.
Directions
- squeeze as needed into your palms and thoroughly spread on both hands.
- rub into skin until dry.
Other Information
- Store at 20 degrees - 25 degrees C (68 degrees to 77 degrees F)
- may discolor fabrics.
Inactive Ingredients
water, propylene glycol, glycerin, fragrance, carbomer, triethanolamine, tocopheryl acetate, red 40, red 33, yellow 5, blue 1
DISTRIBUTED BY:
GREENBRIER INTERNATIONAL, INC.
500 VOLVO PARKWAY, CHESAPEAKE, VA 23320
MADE IN CHINA
| COCONUT LIME SCENTED HAND SANITIZER
ethyl alcohol gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Greenbrier International, Inc. (610322518) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LAB DOUCE BIOTECHNOLOGY HUIZHOU LTD | 554425078 | manufacture(33992-8962) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.