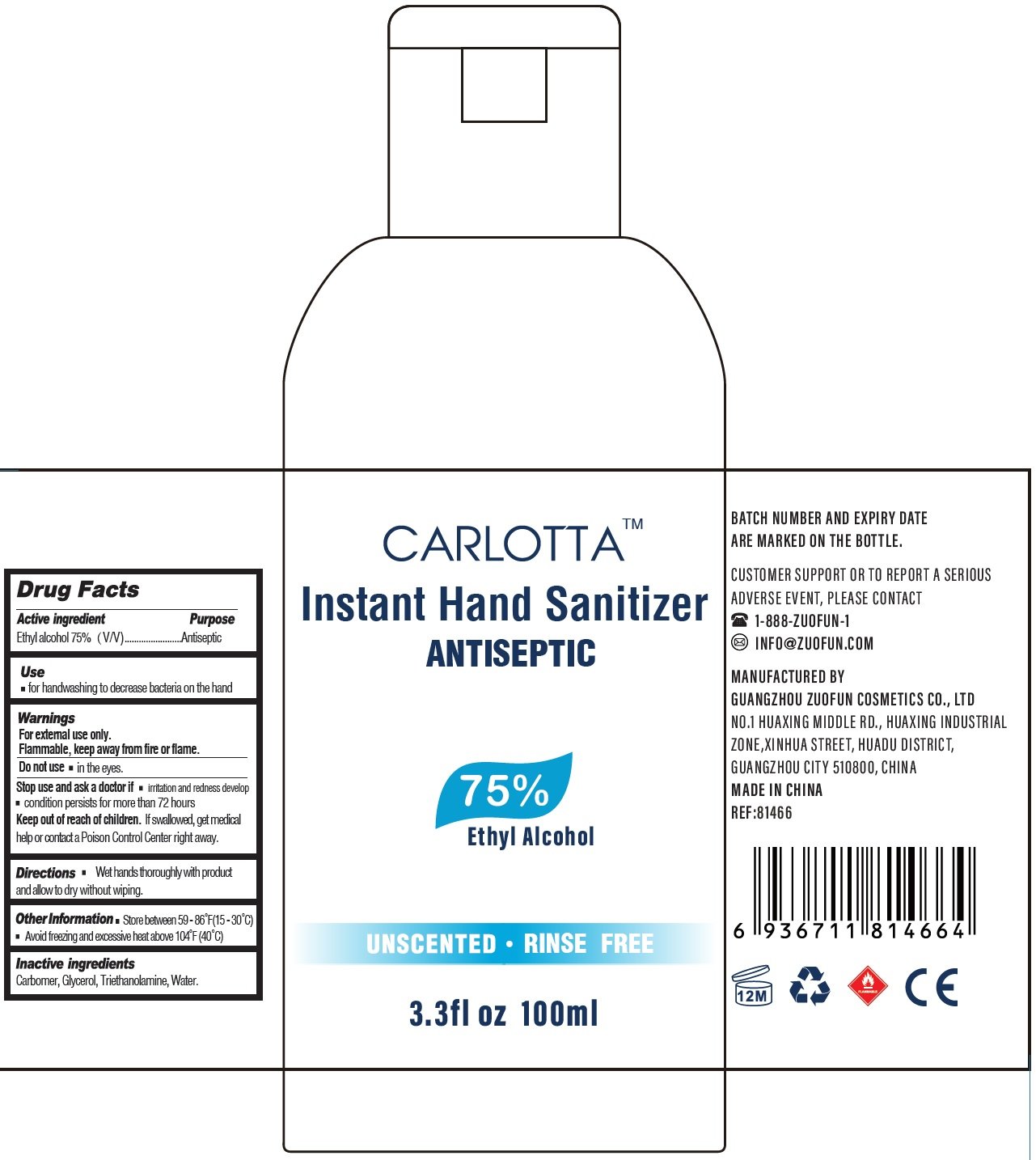
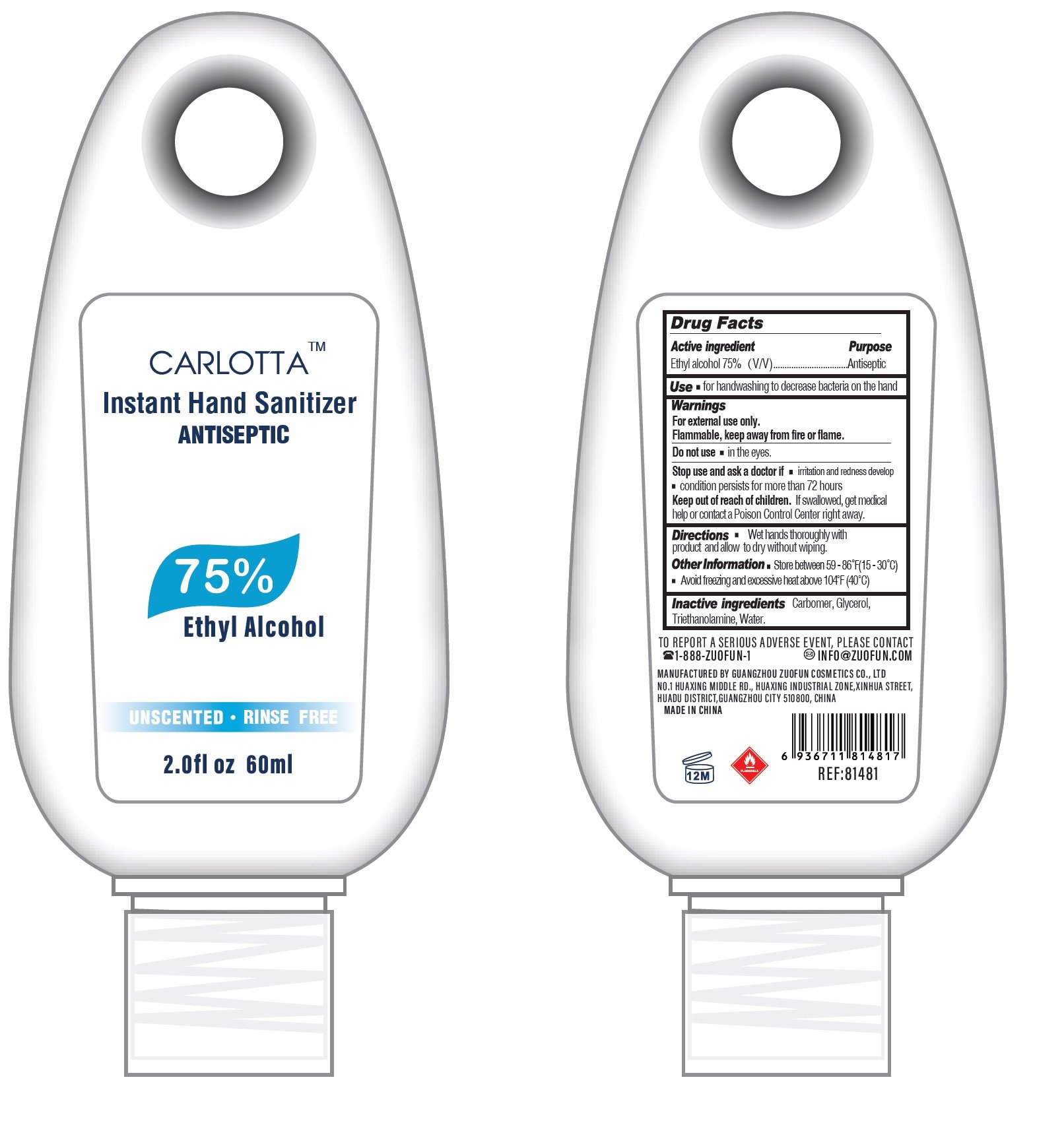
Carlotta Instant Hand Sanitizer
Dosage form: gel
Ingredients: ALCOHOL 0.75mL in 1mL
Labeler: Guangzhou Zuofun Cosmetics Co., Ltd
NDC code: 77027-000
Medically reviewed by Drugs.com. Last updated on Jul 18, 2023.
Drug Facts
Active ingredient
Ethyl alcohol 75%(V/V)
Purpose
Antiseptic
Use
for handwashing to decrease bacteria on the hand
Warnings
For external use only..
Flammable, keep away from fire or flame.
Do not use
in the eyes.
Stop use and ask a doctor if
- irritation and redness develop
- condition persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands thoroughly with product and allow to
Other Information
- Store between 59-86°F (15-30°C)
- Avoid freezing and excessive heat above 104°F (40°C)
Inactive ingredients
Carbomer, Glycerol, Triethanolamine, Water.
| CARLOTTA INSTANT HAND SANITIZER
alcohol gel |
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| Labeler - Guangzhou Zuofun Cosmetics Co., Ltd (530135094) |
Document Id: ab84f335-226b-68a9-e053-2995a90a6c8a
Set id: a4832e4b-2dc3-8362-e053-2995a90ac09c
Version: 6
Effective Time: 20200728
Guangzhou Zuofun Cosmetics Co., Ltd
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.