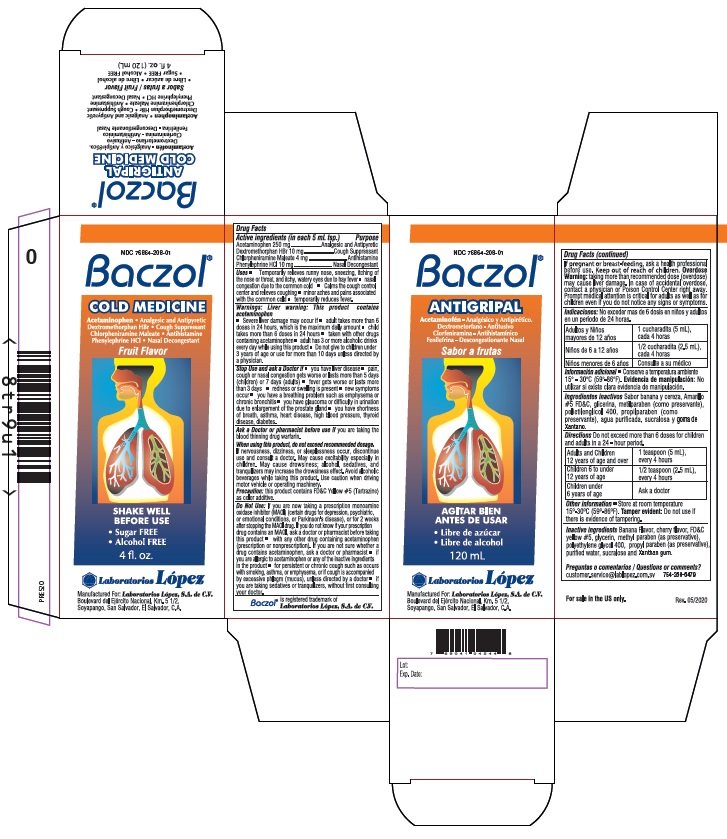
BACZOL COLD MEDICINE
Dosage form: syrup
Ingredients: ACETAMINOPHEN 250mg in 5mL, DEXTROMETHORPHAN HYDROBROMIDE 10mg in 5mL, CHLORPHENIRAMINE MALEATE 4mg in 5mL, PHENYLEPHRINE HYDROCHLORIDE 10mg in 5mL
Labeler: Laboratorios Lopez S.A. de C.V.
NDC code: 76864-208
Medically reviewed by Drugs.com. Last updated on May 10, 2023.
Drug Facts
Active ingredients (in each 5 mL tsp.)
Acetaminophen 250 mg
Dextromethorphan HBr 10 mg
Chlorpheniramine Maleate 4 mg
Phenylephrine HCl 10 mg
Purpose
Acetaminophen .................... Analgesic and Antipyretic
Dextromethorphan HBr ....................Cough Suppressant
Chlorpheniramine Maleate .........................Antihistamine
Phenylephrine HCl ........................ Nasal Decongestant
Uses • Temporarily relieves runny nose, sneezing, itching of
the nose or throat, and itchy, watery eyes due to hay fever • nasal
congestion due to the common cold • Calms the cough control
center and relieves coughing • minor aches and pains associated
with the common cold • temporarily reduces fever.
Warnings: Liver warning: This product contains
acetaminophen
• Severe liver damage may occur if • adult takes more than 6
doses in 24 hours, which is the maximum daily amount •child
takes more than 6 doses in 24 hours • taken with other drugs
containing acetaminophen• adult has 3 or more alcoholic drinks
every day while using this product • Do not give to children
under 3 years of age or use for more than 10 days unless directed by
a physician.
Stop Use and ask a Doctor if • you have liver disease • pain, cough or nasal congestion gets
worse or lasts more than 5 days (children) or 7 days (adults) • fever gets worse or lasts more
than 3 days • redness or swelling is present • new symptoms occur • you have a breathing
problem such as emphysema or chronic bronchitis • you have glaucoma or difficulty in urination
due to enlargement of the prostate gland • you have shortness of breath, asthma, heart disease,
high blood pressure, thyroid disease, diabetes.
Ask a Doctor or pharmacist before use if you are taking the blood thinning drug warfarin.
When using this product, do not exceed recommended dosage. If nervousness, dizziness,
or sleeplessness occur, discontinue use and consult a doctor. May cause excitability especially
in children. May cause drowsiness; alcohol, sedatives, and tranquilizers may increase the
drowsiness effect. Avoid alcoholic beverages while taking this product. Use caution when driving
motor vehicle or operating machinery.
Precaution: this product contains FD&C Yellow #5 (Tartrazine) as color additive.
Do Not Use:If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain
drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2
weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an
MAOI, ask a doctor or pharmacist before taking this product • with any other drug containing
acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist • if you are allergic to acetaminophen or any of the
inactive ingredients in the product • for persistent or chronic cough such as occurs with smoking,
asthma, or emphysema, or if cough is accompanied by excessive phlegm (mucus), unless
directed by a doctor • if you are taking sedatives or tranquilizers, without first consulting your
doctor.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose Warning: taking more than recommended
dose (overdose) may cause liver damage. In case of accidental overdose,
contact a physician or Poison Control Center right away. Prompt medical
attention is critical for adults as well as for children even if you do not notice
any signs or symptoms.
Directions Do not exceed more than 6 doses for children and adults
in a 24 - hour period.
| Adults and Children 12 years of age and over | 1 teaspoon (5 mL) every 4 hours |
| Children 6 to under 12 years of age | 1/2 teaspoon (2.5 mL) every 4 hours |
| Children under 6 years of age | Ask a doctor |
Other information • Store at room temperature 15º-30ºC (59º-86ºF).
Tamper evident: Do not use if there is evidence of tampering.
Inactive ingredients Banana Flavor, cherry flavor, FD&C
yellow #5, glycerin, methyl paraben (as preservative),
polyethylene glycol 400, propyl paraben (as preservative),
purified water, sucralose and Xanthan gum.
Questions or comments?
customer.service@lablopez.com.sv 754-260-6479
NDC 76864-208-01
Baczol®
COLD MEDICINE
Acetaminophen • Analgesic and Antipyretic
Dextromethorphan HBr • Cough Suppressant
Chlorpheniramine Maleate • Antihistamine
Phenylephrine HCl • Nasal Decongestant
Fruit Flavor
For sale in the
US only.
SHAKE WELL
BEFORE USE
• Sugar FREE • Alcohol FREE
4 fl.oz. (120 mL)
Manufactured For: Laboratorios López, S.A. de C.V.
Boulevard del Ejército Nacional, Km. 5 1/2,
Soyapango, San Salvador, El Salvador, C.A.
Baczol® is registered trademark of Laboratorios López, S.A. de C.V.
7 69041 04544 8
Lot: Rev. 05/2020
Exp. Date:
res
| BACZOL COLD MEDICINE
acetaminophen, dextromethorphan hbr, chlorpheniramine maleate, phenylephrine hcl syrup |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Laboratorios Lopez S.A. de C.V. (851259341) |
| Registrant - Laboratorios Lopez S.A. de C.V. (851259341) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.