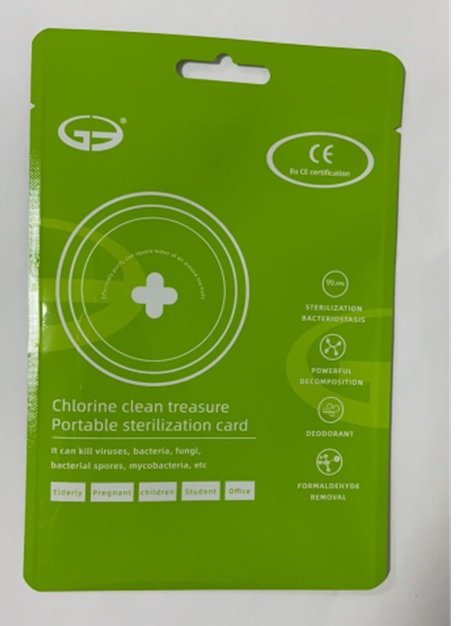
CLO2--Portable sterilization card
Dosage form: powder
Ingredients: CHLORIC ACID 10g in 15g
Labeler: Shenzhen Haiyin Hongye Technology Co., Ltd.
NDC code: 77261-201
Medically reviewed by Drugs.com. Last updated on Apr 29, 2024.
CLO2--Portable sterilization card
Chlorine dioxide 10g/15g. Purpose: Antiseptic
Antiseptic, Portable sterilization card
Portable sterilization card to help reduce bacteria that potentially can cause disease.
For external use only. Flammable. Keep away from heat or flame
- in children less than 2 months of age
- on open skin wounds
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Take it with you, the gas it releases can kill bacteria
- Supervise children under 6 years of age when using this product to avoid swallowing.
- Store between 15-30C (59-86F)
- Avoid freezing and excessive heat above 40C (104F)
Urea nitrate
Magnesium sulfate
Calcium sulphate
Superabsorbent acrylic resin
Beta cyclodextrins
| CLO2--PORTABLE STERILIZATION CARD
clo2 powder |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - Shenzhen Haiyin Hongye Technology Co., Ltd. (419701769) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Shenzhen Haiyin Hongye Technology Co., Ltd. | 419701769 | manufacture(77261-201) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.