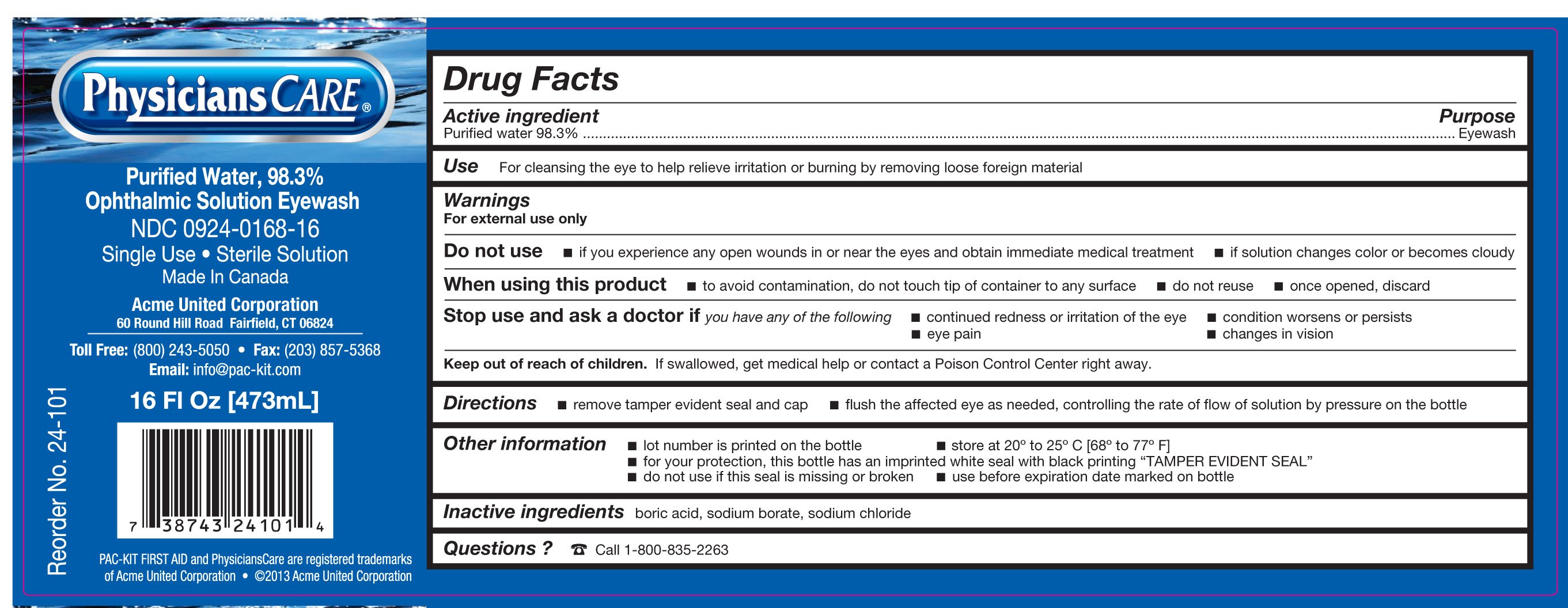
PhysiciansCare Ophthalmic Solution Eyewash Purified Water, 98.3%
Dosage form: solution
Ingredients: WATER 465mL in 473mL
Labeler: Acme United Corporation
NDC code: 0924-0158
Medically reviewed by Drugs.com. Last updated on Sep 4, 2023.
Active Ingredient
Purified Water 98.3%
Purpose
Eyewash
Use
For cleansing the eye to help relieve irritation or burning by removing loose foreign material
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
Stop use and ask a doctor if you have any of the following
- continued redness or irritation of the eye
- condition worsens or persists
- eye pain
- changes in vision
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- remove tamper evident seal and cap
- flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle
Other information
- lot number is printed on the bottle
- store at 20° to 25° C [68° to 77° F]
- for your protection, this bottle has an imprinted white seal with black printing "TAMPER EVIDENT SEAL"
- do not use if this seal is missing or broken
- use before expiration date marked on bottle
Inactive ingredients
boric acid, sodium borate, sodium chloride
Questions? Call 1-800-835-2263
| Labeler - Acme United Corporation (001180207) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Acme United Corporation | 045924339 | relabel(0924-0158), repack(0924-0158) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Acme United Corporation Vancouver Division | 080119599 | relabel(0924-0158), repack(0924-0158) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.