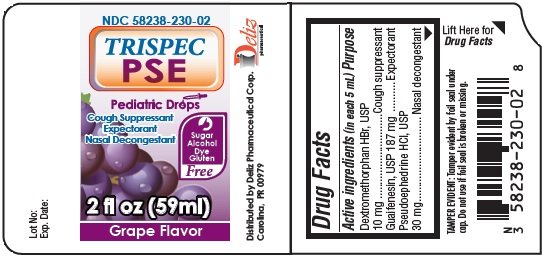
TRISPEC PSE Pediatric Drops Cough Suppressant Expectorant Nasal Decongestant GRAPE Flavor
Dosage form: liquid
Ingredients: DEXTROMETHORPHAN HYDROBROMIDE 10mg in 5mL, GUAIFENESIN 187mg in 5mL, PSEUDOEPHEDRINE HYDROCHLORIDE 30mg in 5mL
Labeler: Deliz Pharmaceutical Corp
NDC code: 58238-230
Medically reviewed by Drugs.com. Last updated on Aug 18, 2023.
Dextromethorphan HBr, USP 10 mg
Guaifenesin, USP 187 mg
Pseudoephedrine HCl, USP 30 mg
Cough suppressant
Expectorant
Nasal decongestant
- temporarily relieves cough due to minor throat and bronchial irritation occuring with a cold or inhaled irritants
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily relieves nasal congestion
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by excessive phlegm (mugus)
- do not exceed recommended dosage
- cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
- symptoms do not improve within 7 days or are acompanied by fever
- nervousness, dizziness, or sleeplessness occur
In case of overdose, get medical help or contact a Poison Control Center right away.
- For children under 6 years of age, use enclosed dropper
- do not use enclosed dropper for any other drug product
| Children 2 to under 6 years of age:
| 2½ mL every 4 hours not to exceed 10 mL in 24 hours, or as directed by a doctor.
|
| Children under 2 years of age:
| Consult a doctor.
|
- Store at room temperature 15°C-30°C (59°F-86°F)
Bitter Mask, Citric Acid Anhydrous, Glycerin, Grape Flavor, Propylene Glycol, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol
Call 1-787-701-3312. You may also report serious side effects to this phone number.
| TRISPEC PSE PEDIATRIC DROPS COUGH SUPPRESSANT EXPECTORANT NASAL DECONGESTANT GRAPE FLAVOR
dextromethorphan hydrobromide, guaifenesin, pseudoephedrine hydrochloride liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Deliz Pharmaceutical Corp (826391138) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Woodfield Pharmaceutical, LLC | 079398730 | manufacture(58238-230) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.