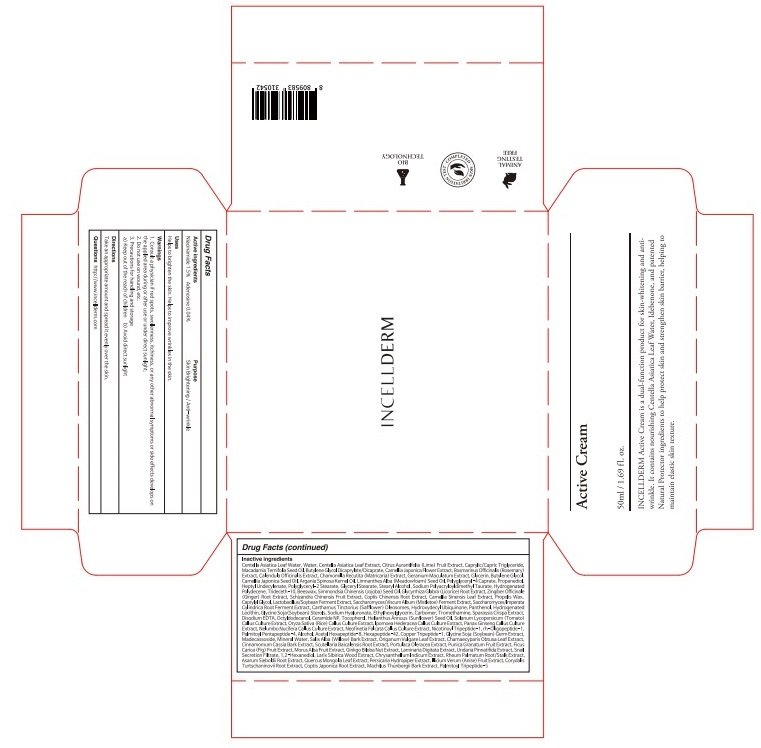
INCELLDERM ACTIVE
Dosage form: cream
Ingredients: Niacinamide 0.75g in 50mL, Adenosine 0.02g in 50mL
Labeler: Riman Co., Ltd.
NDC code: 72650-010
Medically reviewed by Drugs.com. Last updated on Aug 10, 2023.
Niacinamide 1.5%
Adenosine 0.04%
Centella Asiatica Leaf Water, Water, Centella Asiatica Leaf Extract, Citrus Aurantlfolia (Lime) Fruit Extract, Caprylic/Capric Triglyceride, Macadamia Ternifolia Seed Oil, Butylene Glycol Dicaprylate/Dicaprate, Camellia Japonica Flower Extract, Rosmarinus Officinalis (Rosemary) Extract, Calendula
Officinalis Extract, Chamomilla Recutita (Matricaria) Extract, Geranium Maculatum Extract, Glycerin, Butylene Glycol, Camellia Japonica Seed Oil, Argania Spinosa Kernel Oil, Limnanthes Alba (Meadowfoam) Seed Oil, Polyglyceryl-4 Caprate, Propanediol, Heptyl Undecylenate, Polyglyceryl-2 Stearate, Glyceryl Stearate, Stearyl Alcohol, Sodium Polyacryloyldimethyl Taurate, Hydrogenated Polydecene, Trideceth-10, Beeswax, Simmondsia Chinensis (Jojoba) Seed Oil, Glycyrrhiza Glabra (Licorice) Root Extract, Zingiber Officinale (Ginger) Root Extract, Schizandra Chinensis Fruit Extract, Coptis Chinensis Root Extract, Camellia Sinensis Leaf Extract, Propolis Wax, Caprylyl Glycol, Lactobacillus/Soybean Ferment Extract, Saccharomyces/Viscum Album (Mistletoe) Ferment Extract, Saccharomyces/Imperata Cylindrica Root Ferment Extract, Carthamus Tinctorius (Safflower) Oleosomes, Hydroxydecyl Ubiquinone, Panthenol, Hydrogenated Lecithin, Glycine Soja(Soybean) Sterols, Sodium Hyaluronate, Ethylhexylglycerin, Carbomer, Tromethamine, Sparassis Crispa Extract, Disodium EDTA, Octyldodecanol, Ceramide NP, Tocopherol, Helianthus Annuus (Sunflower) Seed Oil, Solanum Lycopersicum (Tomato) Callus Culture Extract, Oryza Sativa (Rice) Callus Culture Extract, Ipomoea Hederacea Callus Culture Extract, Panax Ginseng Callus Culture Extract, Nelumbo Nucifera Callus Culture Extract, Neofinetia Falcata Callus Culture Extract, Nicotinoyl Tripeptide-1, rh-Oligopeptide-1, Palmitoyl Pentapeptide-4, Alcohol, Acetyl Hexapeptide-8, Hexapeptide-42, Copper Tripeptide-1, Glycine Soja (Soybean) Germ Extract, Madecassoside, Mineral Water, Salix Alba (Willow) Bark Extract, Origanum Vulgare Leaf Extract, Chamaecyparis Obtusa Leaf Extract, Cinnamomum Cassia Bark Extract, Scutellaria Baicalensis Root Extract, Portulaca Oleracea Extract, Punica Granatum Fruit Extract, Ficus Carica (Fig) Fruit Extract, Morus Alba Fruit Extract, Ginkgo Biloba Nut Extract, Laminaria Digitata Extract, Undaria Pinnatifida Extract, Snail Secretion Filtrate, 1,2-Hexanediol, Larix Sibirica Wood Extract, Chrysanthellum Indicum Extract, Rheum Palmatum Root/Stalk Extract, Asarum Sieboldii Root Extract, Quercus Mongolia Leaf Extract, Persicaria Hydropiper Extract, Illicium Verum (Anise) Fruit Extract, Corydalis Turtschaninovii Root Extract, Coptis Japonica Root Extract, Machilus Thunbergii Bark Extract, Palmitoyl Tripeptide-5
Skin Brightening
Anti-wrinkle
1. Consult a physician if red spots, swollenness, itchiness, or any other abnormal symptoms or side effects develops on the applied area during or after use or under direct sunlight.
2. Do not use on wound, etc.
3. Precautions for handling and storage
a) Keep out of the reach of children
b) Avoid direct sunlight
KEEP OUT OF REACH OF CHILDREN
Helps to brighten the skin.
Helps to improve wrinkles in the skin.
Take an appropriate amount and spread it evenly over the skin.
Please put the appropriate amount on the skin and wait (daub).
| INCELLDERM ACTIVE
niacinamide, adenosine cream |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Riman Co., Ltd. (694893735) |
| Registrant - Riman Co., Ltd. (694893735) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| HANSOLBIOTECH | 694455165 | manufacture(72650-010) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.