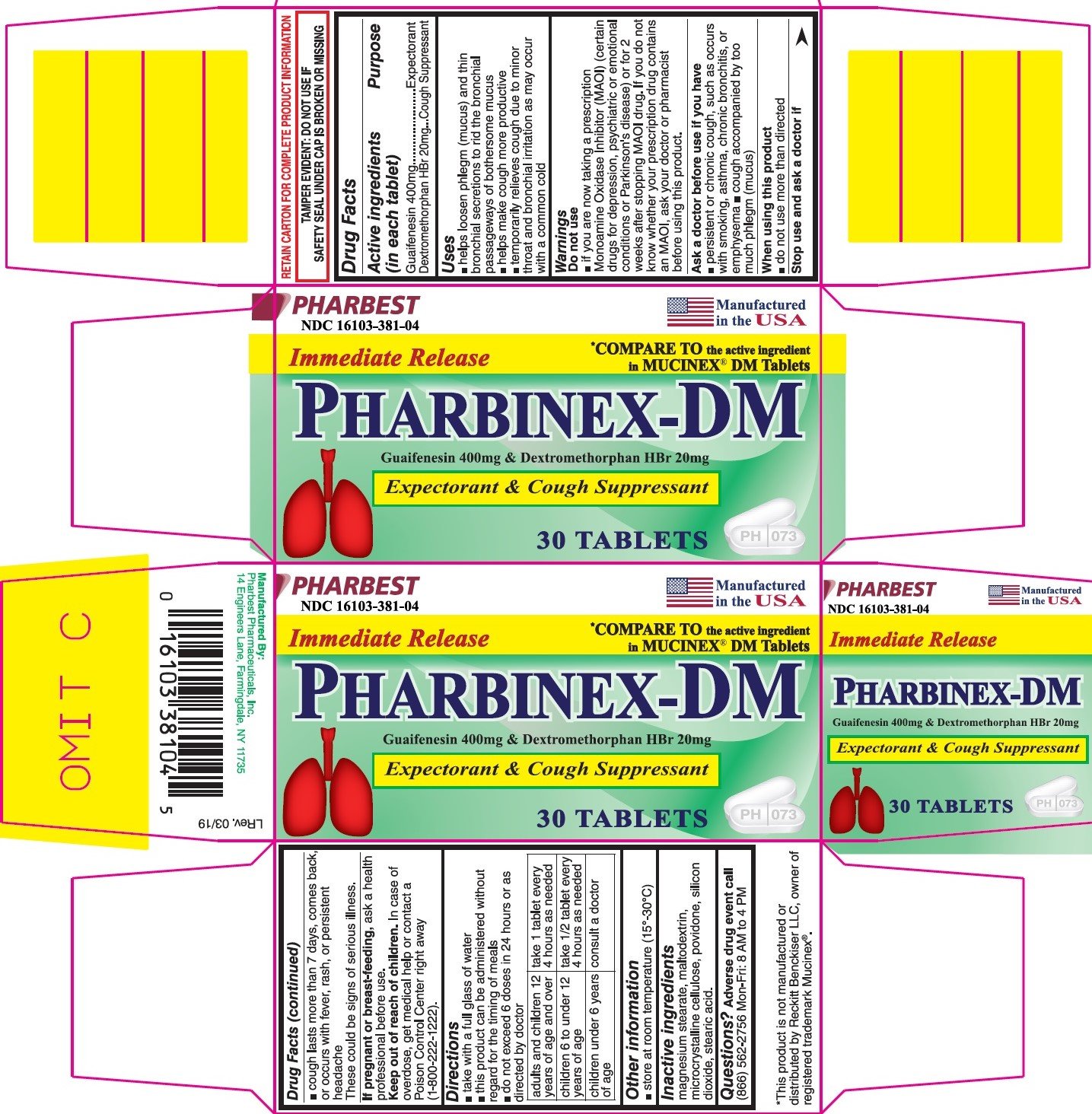
PHARBINEX-DM
Dosage form: tablet
Ingredients: GUAIFENESIN 400mg, DEXTROMETHORPHAN HYDROBROMIDE 20mg
Labeler: Pharbest Pharmaceuticals, Inc.
NDC code: 16103-381
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
Guaifenesin 400mg
Dextromethorphan HBr 20mg
Expectorant
Cough suppressant
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus
- helps make cough more productive
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a common cold
Do not use
- if you are now taking a prescription Monoamine Oxidase Inhibitor (MAOI)(certain drugs for depression, psychiatric or emotional conditions or Parkinson's disease) or for 2 weeks after stopping MAOI drug. If you do not know whether your prescription drug contains MAOI, ask your doctor or pharmacist before using this product.
- persistent or chronic cough, such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
- do not use more than directed
- cough lasts more than 7 days, comes back, or occure with fever, rash or persistent headache
These could be signs of serious illness.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- do not exceed 6 doses in 24 hours or as directed by doctor
|
adults and children 12 years of age and over |
take 1 tablet every 4 hours as needed |
|
children 6 to under 12 years of age |
take ½ tablet every 4 hours as needed |
|
children under 6 years of age |
consult a doctor |
- store at room temperature (15°-30°C)
magnesium stearate, maltodextrin, microcrystalline celluclose, povidone, silicon dioxide, stearic acid.
Adverse drug event call (866) 562-2756 Mon-Fri: 8 AM to 4 PM
| PHARBINEX-DM
guaifenesin 400mg and dextromethorphan hbr 20mg tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pharbest Pharmaceuticals, Inc. (557054835) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pharbest Pharmaceuticals, Inc. | 557054835 | manufacture(16103-381), analysis(16103-381), pack(16103-381), label(16103-381) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.