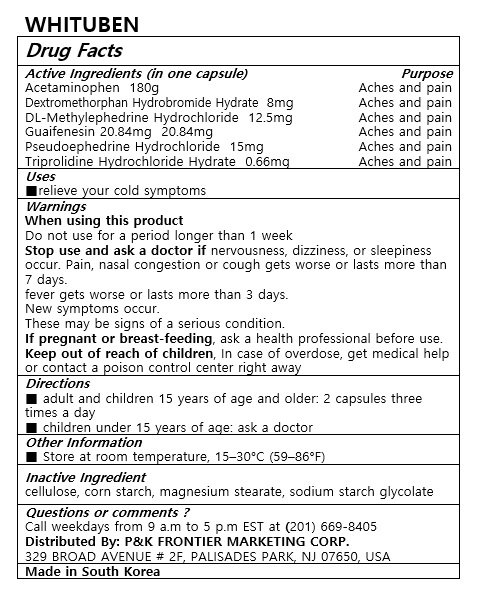
WHITUBEN
Dosage form: capsule
Ingredients: ACETAMINOPHEN 180mg, GUAIFENESIN 20.84mg, PSEUDOEPHEDRINE HYDROCHLORIDE 15mg, METHYLEPHEDRINE HYDROCHLORIDE, (+/-)- 12.5mg, TRIPROLIDINE HYDROCHLORIDE 0.66mg, DEXTROMETHORPHAN HYDROBROMIDE 8mg
Labeler: OASIS TRADING
NDC code: 72689-0037
Medically reviewed by Drugs.com. Last updated on Mar 8, 2024.
Acetaminophen 180mg
Dextromethorphan Hydrobromide Hydrate 8mg
DL-Methylephedrine Hydrochloride 12.5mg
Guaifenesin 20.84mg
Pseudoephedrine Hydrochloride 15mg
Triprolidine Hydrochloride Hydrate 0.66mg
relieve your cold symptoms
Keep out of reach of children
■ adult and children 15 years of age and older: 2 capsules three times a day
■ children under 15 years of age: ask a doctor
Warnings
When using this product
Do not use for a period longer than 1 weeks
Stop use and ask a doctor if nervousness, dizziness, or sleepness occur. Pain, nasal congestion or cough gets worse or lasts more than 7 days.
fever gets worse or lasts more than 3 days
New symptoms occur
These may be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children, In case of overdose, get medical help or contact a poison control center right away
cellulose, corn starch, magnesium stearate, sodium starch glycolate
For oral use only
| WHITUBEN
acetaminophen, dextromethorphan hydrobromide hydrate, guaifenesin, pseudoephedrine hydrochloride, triprolidine hydrochloride hydrate capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - OASIS TRADING (689991468) |
| Registrant - OASIS TRADING (689991468) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OASIS TRADING | 689991468 | manufacture(72689-0037), label(72689-0037) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.