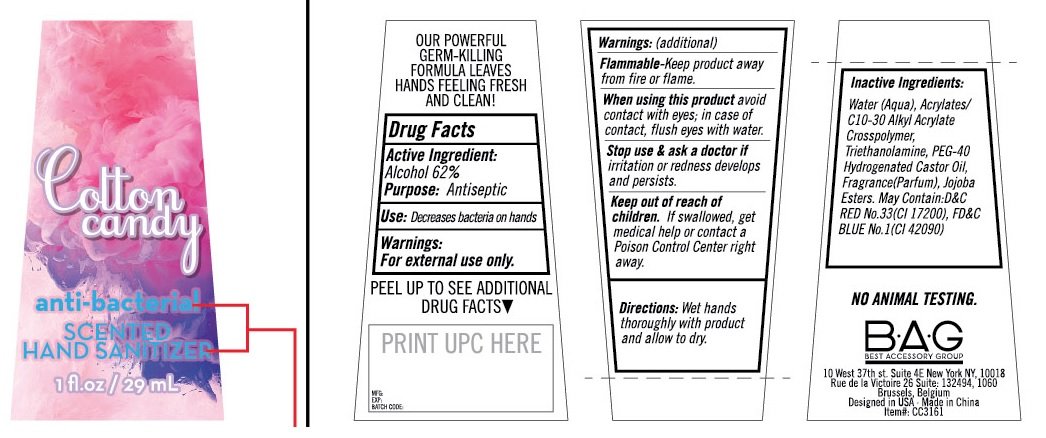
Cotton candy anti-bacterial SCENTED HAND SANITIZER
Dosage form: solution
Ingredients: ALCOHOL 62mL in 100mL
Labeler: Best Accessory Group
NDC code: 60533-003
Medically reviewed by Drugs.com. Last updated on Aug 3, 2023.
OUR POWERFUL GERM-KILLING FORMULA LEAVES HANDS FEELING FRESH AND CLEAN !
Drug Facts
Active Ingredient:
Alcohol 62%
Purpose: Antiseptic
Use: Decreases bacteria on hands.
Warnings:
For External Use Only.
Flammable -Keep product away from fire or flame.
When using this product avoid contact with eyes; in case of contact, flush eyes with water.
Stop use & ask a doctor if irritation or redness develops and persists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions: Wet hands thoroughly with product and allow to dry.
Inactive Ingredients:
Water(Aqua), Acrylates/C10-30 Alkyl Acrylate Crosspolymer,Triethanolamine, PEG-40 Hydrogenated Castor Oil, Fragrance (Perfume), Jojoba Esters. May Contain: D&C RED No.33 (CI 17200), FD&C BLUE No. 1 (CI 42090)
NO ANIMAL TESTING
B•A•G
BEST ACCESSORY GROUP
10 West 37th st. Suite 4E New York NY, 10018
Rue de la Victoire 26 Suite: 132494, 1060
Brussels, Belgium
Designed in USA. Made in China
Item#: CC3161
| COTTON CANDY ANTI-BACTERIAL SCENTED HAND SANITIZER
alcohol solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Best Accessory Group (078712219) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.