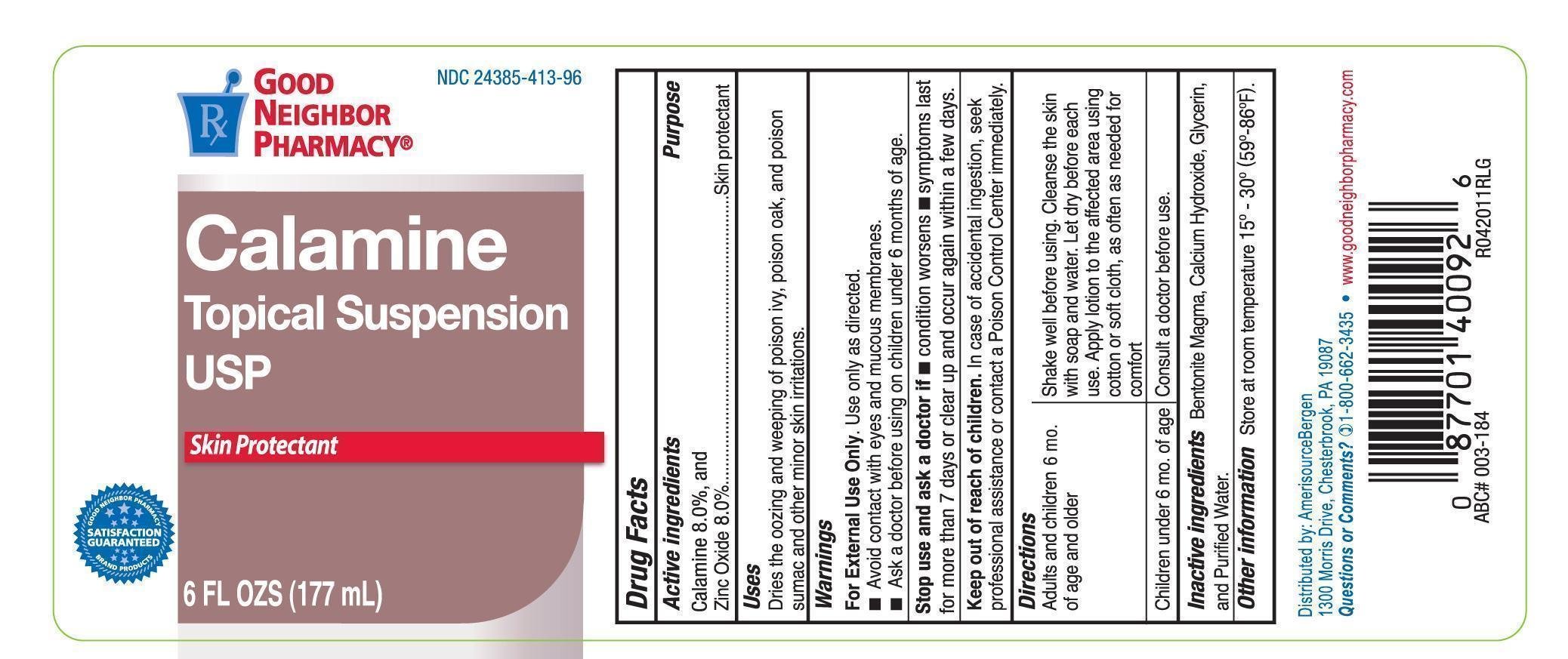
GNP Calamine Topical Suspension
Dosage form: lotion
Ingredients: ZINC OXIDE 160mg in 1mL
Labeler: Amerisource Bergen
NDC code: 24385-413
Medically reviewed by Drugs.com. Last updated on Jun 12, 2023.
Drug Facts
Calamine 8%
Zinc Oxide 8%
Skin Protectant
Skin Protectant
Dries the oozing and weeping of poison ivy, poison oak, and poison sumac and other skin irritations.
For external use only. Use only as directed.
Avoid contat with eyes and moucous membranes.
before using on children under 6 years of age.
Stop use and ask a doctor if
- Condition worsens
- Symptoms last for more than 7 days or clear up and occur again within a few days.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Adults and children 6 mo. of age and older: Shake well before using. Cleanse the skin with soap and water. Let dry before each use. Apply lotion to the affected area using cotton or soft cloth, as often as needed for comfort.
Children under 6 mo. of age: Consult a doctor before use.
Store at room temperature 15-30C (59-86F)
Bentonite Magma, Calcium Hydroxide, Glycerin, and Purified Water.
Distributed by: AmrisourceBergen
1300 Morris Drive, Chesterbrooke, PA 19807
| GNP CALAMINE TOPICAL SUSPENSION
calamine 8% and zinc oxide 8% lotion |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - Amerisource Bergen (007914906) |
| Registrant - Humco Holding Group, Inc. (825672884) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Humco Holding Group, Inc. | 825672884 | analysis(24385-413), manufacture(24385-413), pack(24385-413), label(24385-413) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.