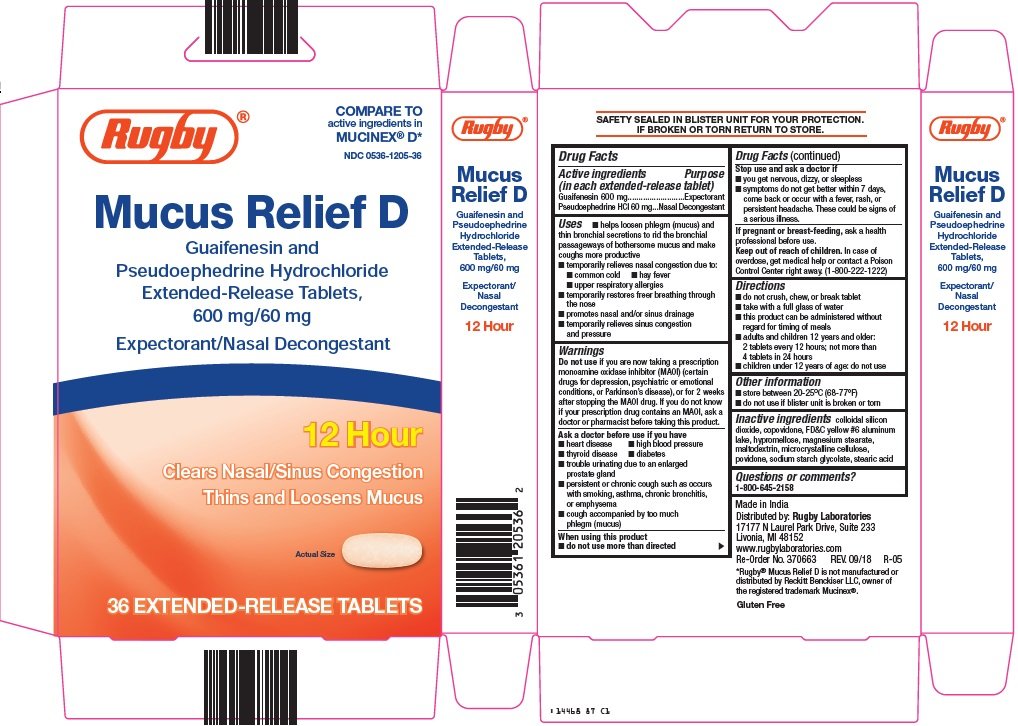
rugby mucus relief d
Dosage form: tablet, extended release
Ingredients: GUAIFENESIN 600mg, PSEUDOEPHEDRINE HYDROCHLORIDE 60mg
Labeler: Rugby Laboratories
NDC code: 0536-1205
Medically reviewed by Drugs.com. Last updated on Feb 5, 2024.
Guaifenesin 600 mg
Pseudoephedrine HCl 60 mg
Expectorant
Nasal Decongestant
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
- •
- temporarily restores freer breathing through the nose
- •
- promotes nasal and/or sinus drainage
- •
- temporarily relieves sinus congestion and pressure
now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
- •
- do not use more than directed
- •
- you get nervous, dizzy, or sleepless
- •
- symptoms do not get better within 7 days, come back or occur with a fever, rash, or persistent headache. These could be signs of a serious illness.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for timing of meals
- •
- adults and children 12 years and older: 2 tablets every 12 hours; not more than 4 tablets in 24 hours
- •
- children under 12 years of age: do not use
- •
- store between 20-25°C (68-77°F)
- •
- do not use if blister unit is broken or torn
colloidal silicon dioxide, copovidone, FD&C yellow #6 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
1-800-645-2158
COMPARE TO active ingredients in MUCINEX® D
Mucus Relief D
Guaifenesin and Pseudoephedrine Hydrochloride
Extended-Release Tablets, 600 mg/60 mg
Expectorant/Nasal Decongestant
12 Hour
Clears Nasal/Sinus Congestion
Thins and Loosens Mucus
Actual Size
36 EXTENDED-RELEASE TABLETS
| RUGBY MUCUS RELIEF D
guaifenesin, pseudoephedrine hydrochloride tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.