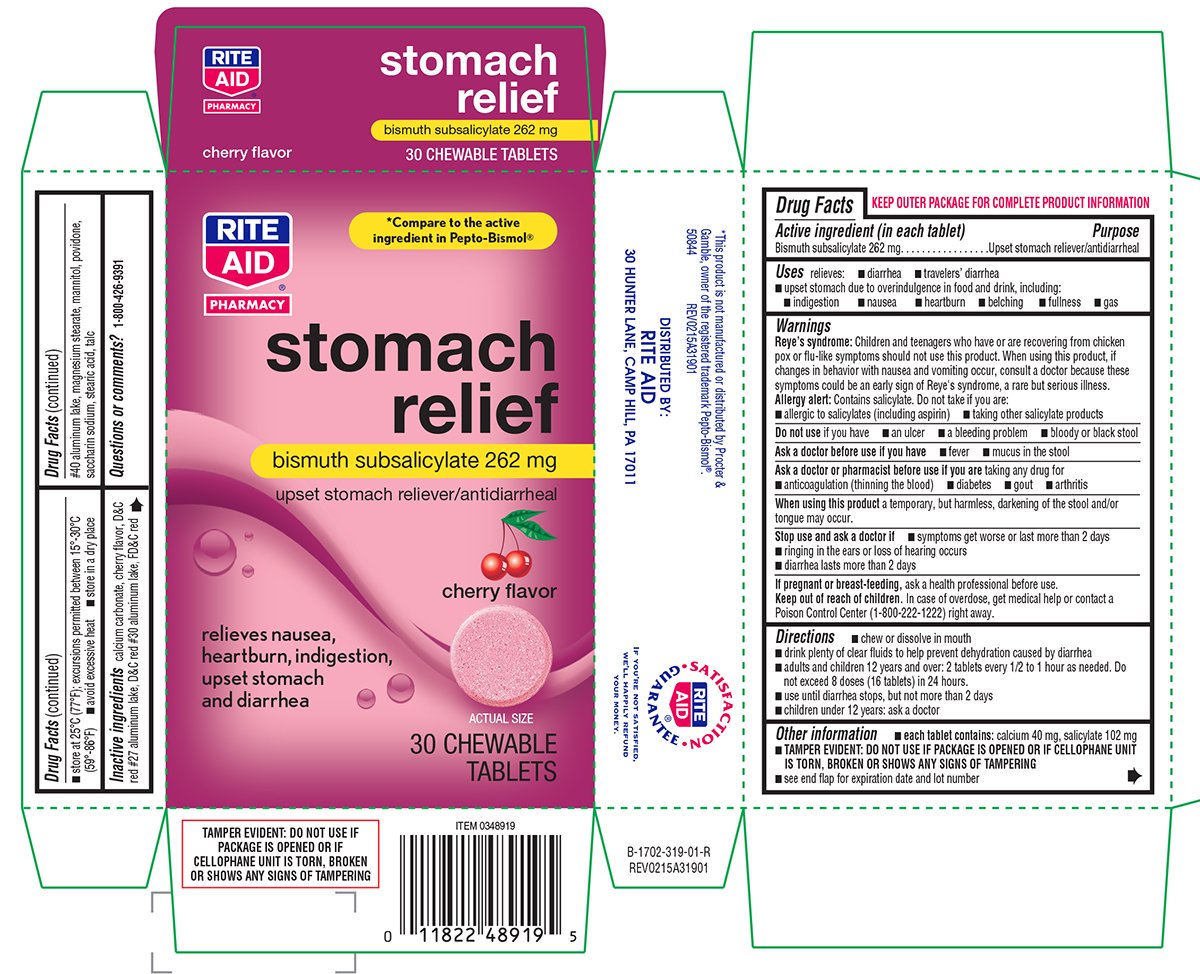
Stomach Relief
Dosage form: tablet, chewable
Ingredients: BISMUTH SUBSALICYLATE 262mg
Labeler: Rite Aid Corporation
NDC code: 11822-0319
Medically reviewed by Drugs.com. Last updated on Apr 10, 2024.
Bismuth subsalicylate 262 mg
Upset stomach reliever/antidiarrheal
relieves:
- diarrhea
- travelers' diarrhea
- upset stomach due to overindulgence in food and drink, including:
- indigestion
- nausea
- heartburn
- belching
- fullness
- gas
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are:
- allergic to salicylates (including aspirin)
- taking other salicylate products
if you have
- an ulcer
- a bleeding problem
- bloody or black stool
- fever
- mucus in the stool
taking any drug for
- anticoagulation (thinning the blood)
- diabetes
- gout
- arthritis
a temporary, but harmless, darkening of the stool and/or tongue may occur.
- symptoms get worse or last more than 2 days
- ringing in the ears or loss of hearing occurs
- diarrhea lasts more than 2 days
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- chew or dissolve in mouth
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed. Do not exceed 8 doses (16 tablets) in 24 hours.
- use until diarrhea stops, but not more than 2 days
- children under 12 years: ask a doctor
- each tablet contains: calcium 40 mg, salicylate 102 mg
-
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF CELLOPHANE UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
- see end flap for expiration date and lot number
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- avoid excessive heat
- store in a dry place
calcium carbonate, cherry flavor, D&C red #27 aluminum lake, D&C red #30 aluminum lake, FD&C red #40 aluminum lake, magnesium stearate, mannitol, povidone, sodium saccharin, stearic acid, talc
RITE
AID®
PHARMACY
*Compare to the active ingredient in Pepto-Bismol®
stomach
relief
bismuth subsalicylate 262 mg
upset stomach reliever/antidiarrheal
cherry flavor
relieves nausea,
heartburn, indigestion,
upset stomach
and diarrhea
ACTUAL SIZE
30 CHEWABLE TABLETS
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF CELLOPHANE UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
DISTRIBUTED BY:
RITE AID
30 HUNTER LANE, CAMP HILL, PA 17011
*This product is not manufactured or distributed by Procter & Gamble, owner of the registered trademark Pepto-Bismol®.
50844 REV0215A31901
Rite Aid 44-319
| STOMACH RELIEF
bismuth subsalicylate tablet, chewable |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Rite Aid Corporation (014578892) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK(11822-0319) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE(11822-0319) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 868734088 | PACK(11822-0319) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867837 | PACK(11822-0319) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 967626305 | PACK(11822-0319) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
