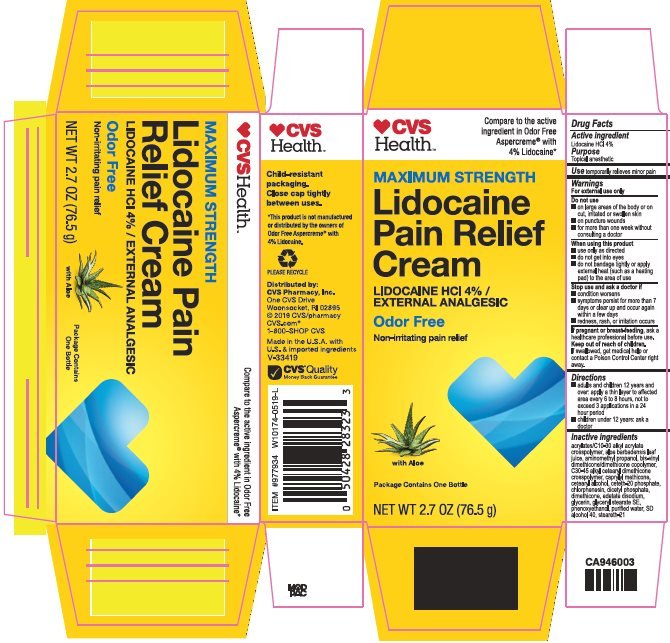
CVS Health Lidocaine Pain Relief
Dosage form: cream
Ingredients: LIDOCAINE HYDROCHLORIDE 40mg in 1g
Labeler: CVS Pharmacy
NDC code: 69842-980
Medically reviewed by Drugs.com. Last updated on Nov 23, 2023.
Lidocaine HCl 4%
Lidocaine HCl - Topical anesthetic
temporarily relieves minor pain
For external use only
- •
- on large areas of the body or on cut, irritated or swollen skin
- •
- on puncture wounds
- •
- for more than one week without consulting a doctor
- •
- use only as directed
- •
- do not get into eyes
- •
- do not bandage tightly or apply external heat (such as a heating pad) to the area of use
- •
- condition worsens
- •
- symptoms persist for more than 7 days or clear up and occur again within a few days
- •
- redness, rash, or irritation occurs
ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
- •
- adults and children 12 years and over: apply a thin layer to affected area every 6 to 8 hours, not to exceed 3 applications in a 24 hour period
- •
- children under 12 years: ask a doctor
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, aminomethyl propanol, bis-vinyl dimethicone/dimethicone copolymer, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, chlorphenesin, dicetyl phosphate, dimethicone, edetate disodium, glycerin, glyceryl stearate SE, phenoxyethanol, purified water, SD alcohol 40, steareth-21
| CVS HEALTH LIDOCAINE PAIN RELIEF
lidocaine hcl cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.