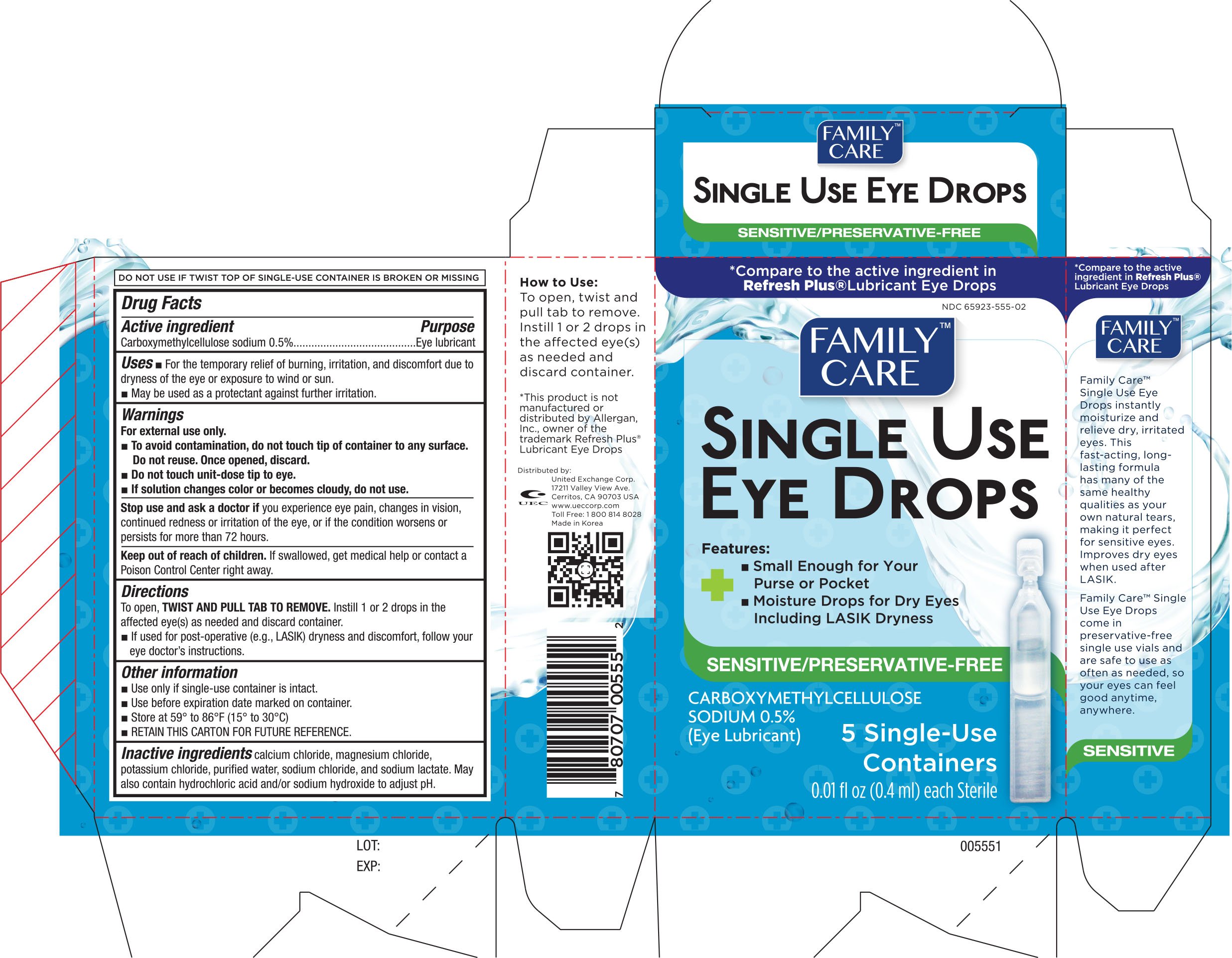
FAMILY CARE LUBRICANT SINGLE USE EYE
Dosage form: solution/ drops
Ingredients: CARBOXYMETHYLCELLULOSE SODIUM 5mg in 1mL
Labeler: UNITED EXCHANGE CORP
NDC code: 65923-555
Medically reviewed by Drugs.com. Last updated on Aug 21, 2023.
Active ingredient Purpose
Carboxymethylcellulose Sodium 0.5%......................................Eye Lubricant
Uses
- For the temporary relief of burning and discomfort due to dryness of the eye or exposure to wind or sun
- May be used as a protectant against further irritation
Warnings
For external use only
- To avoid contamination, do not touch tip of container to any surface
Do not reuse. Once opened, discard
- Do not touch unit-dose tip of eye
- if solution changes color or becomes cloudy, do not use
Do not reuse. Once opened, discard
- Do not touch unit-dose tip of eye
- if solution changes color or becomes cloudy, do not use
Stop use and ask a doctor if you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
To open, TWIST AND PULL TAB TO REMOVE. Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
- If used for post-operative (e.g., LASIK) dryness and discomfort, follow your eye doctor's instructions.
Other information
- Use only if single-use container is intact.
- Use before expiration date marked on container.
- Store at 59° to 86°F (15° to 30°C)
- RETAIN THIS CARTON FOR FUTURE REFERENCE.
calcium chloride, magnesium chloride, potassium chloride, purified water, sodium chloride, and sodium lactate. May also contain hydrochloric acid and/or sodium hydroxide to adjust pH.
Distributed by:
UNITED EXCHANGE CORP.
17211 Valley View Ave.
Cerritos, CA 90703 USA
MADE IN KOREA
| FAMILY CARE LUBRICANT SINGLE USE EYE
carboxymethylcellulose sodium solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - UNITED EXCHANGE CORP (840130579) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.