Promescent
Dosage form: spray
Ingredients: Lidocaine 10g in 100g
Labeler: Absorption
NDC code: 55636-590
Medically reviewed by Drugs.com. Last updated on Mar 6, 2024.
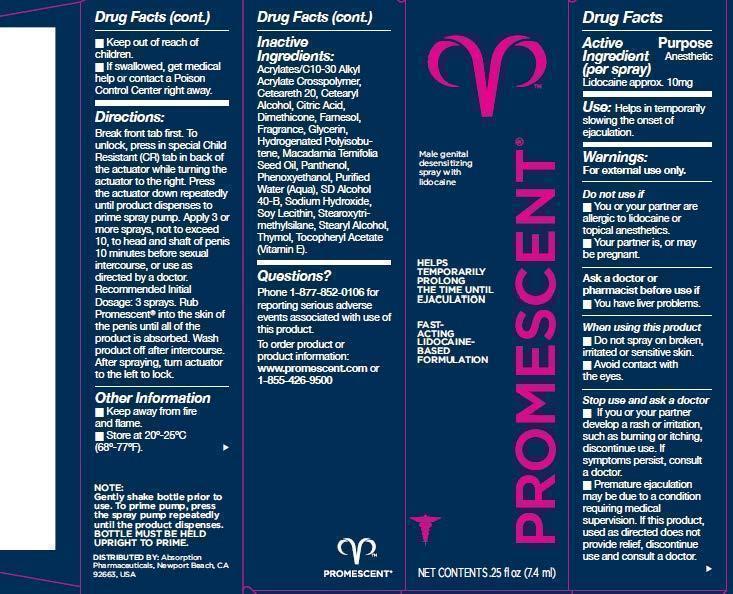
Lidocaine approx. 10mg
Anesthetic
If swallowed, get medical help or contact a Poison Control Center right away
Helps in temporarily slowing the onset of ejaculation.
For external use only.
Do not use if
- You or your partner are allergic to lidocaine or topical anesthetics.
- Your partner is, or may be pregnant.
Ask a doctor or pharmacist before use if
- You have liver problems.
When using this product
- Do not spray on broken, irritated or sensitive skin.
- Avoid contact with the eyes.
Stop use and ask a doctor
- If you or your partner develop a rash or irritation, such as burning or itching, dicontinue use. If symptoms persist, consult a doctor.
- Premature ejaculation may be due to a condition requiring medical supervision. If this product, used as directed does not provide relief, discontinue use and consult a doctor.
Break front tab first. To unlock, press in special Child Resistant (R) tab in back of the actuator while turning the actuator to the right. Press the actuator down repeatedly until product dispenses to prime spray pump. Apply 3 or more sprays, not to exceed 10, to head and shaft of penis 10 minutes before sexual intercourse, or use as directed by a doctor. Recommended Initial Dosage: 3 sprays. Rub Promescent into the skin of the penis until all of the product is absorbed. Wash product off after intersourse. After spraying, turn actuator to the left to lock.
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Ceteareth-20, Cetearyl Alcohol, Citric Acid, Dimethicone, Farnesol, Fragrance, Glycerin, Hydrogenated Polyisobutene, Macadamia Ternifolia Seed Oil, Panthenol, Phenoxyethanol, Purified Water (Aqua), SD Alcohol 40-B, Sodium Hydroxide, Soy Lecithin, Stearoxytrimethylsilane, Stearyl Alcohol, Thymol, Tocopheryl Acetate (Vitamin E).
| PROMESCENT
lidicaine spray |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Absorption (014937753) |
| Registrant - Swiss-American CDMO, LLC (080170933) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Swiss-American CDMO, LLC | 080170933 | manufacture(55636-590), label(55636-590), pack(55636-590) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.