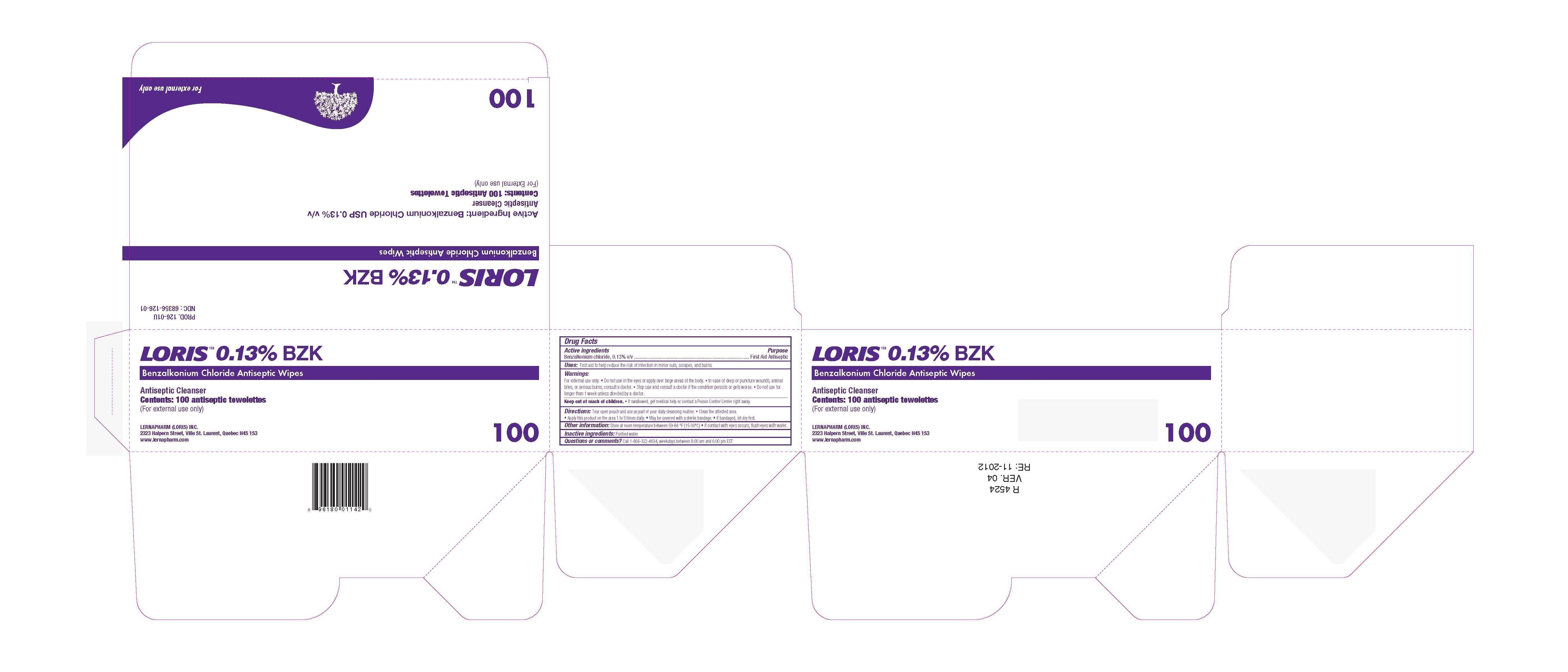
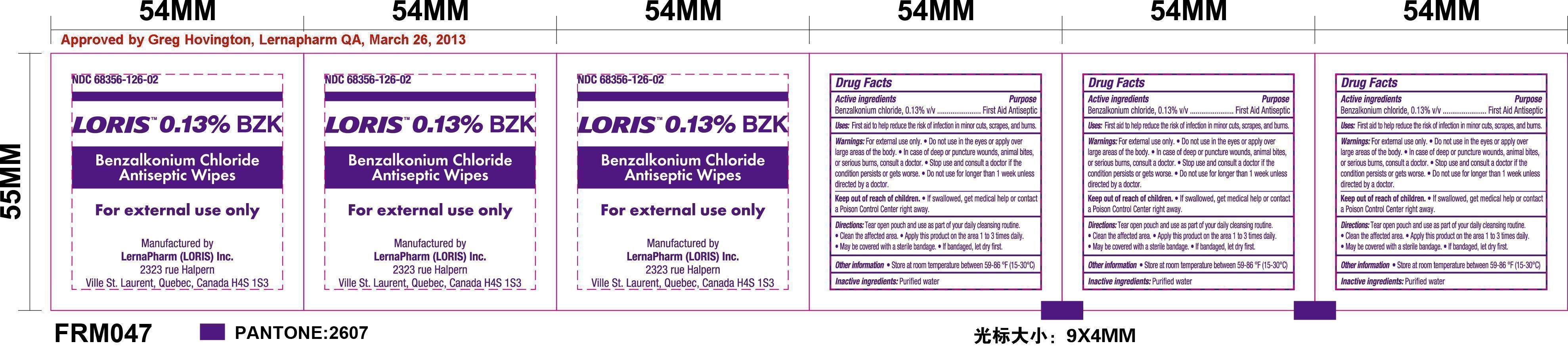
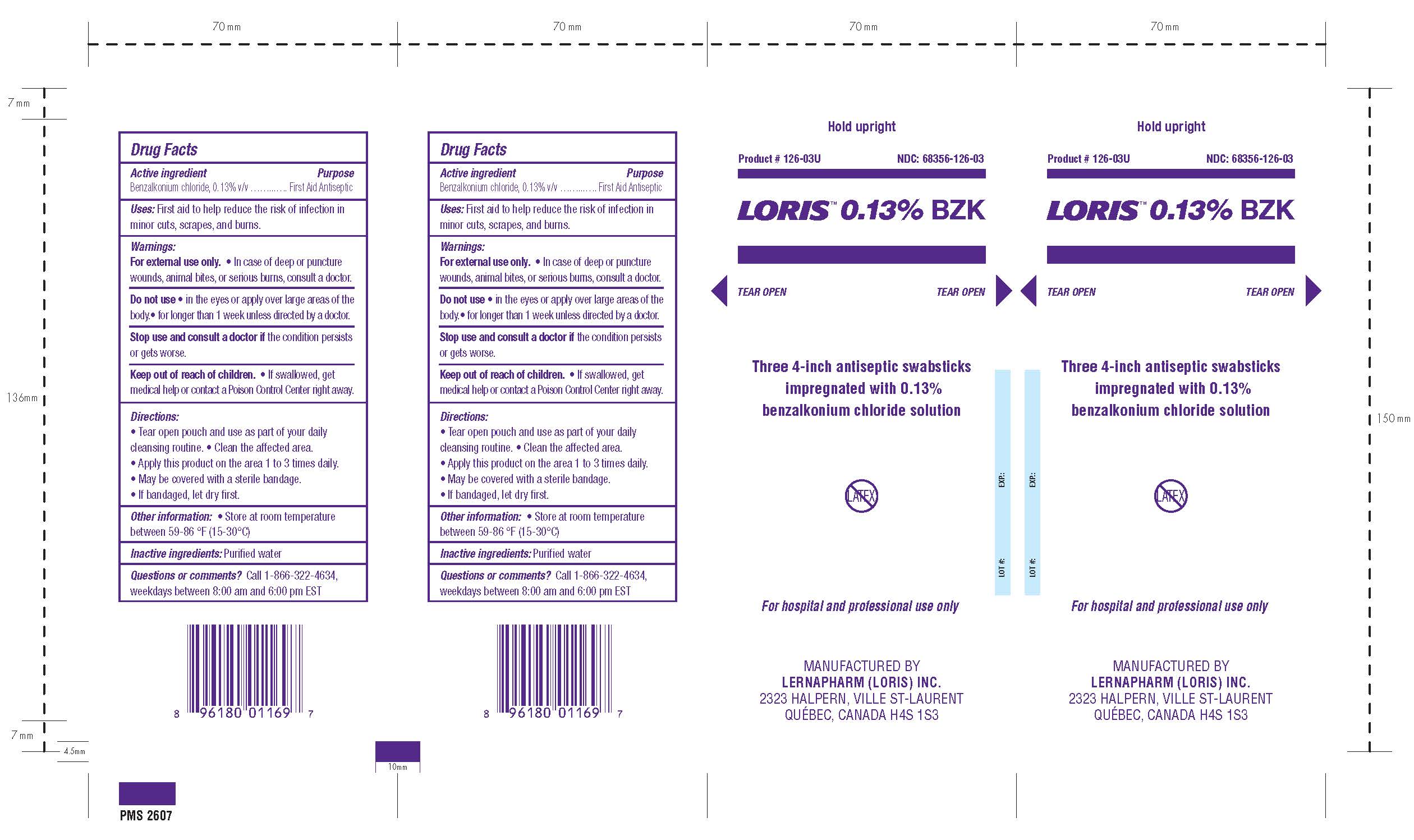
Loris BZK Antiseptic Wipes
Dosage form: swab
Ingredients: BENZALKONIUM CHLORIDE 0.13g in 100g
Labeler: LernaPharm Loris Inc.
NDC code: 68356-126
Medically reviewed by Drugs.com. Last updated on Aug 14, 2023.
Benzalkonium chloride, 0.13% v/v
First Aid Antiseptic
First aid to help reduce the risk of infection in minor cuts, scrapes, and burns.
For External Use Only
- Do not use in the eyes or apply over large areas of the body.
- In case of deep or puncture wounds, animal bites, or serious burns, consult a doctor.
- Stop use and consult a doctor if the condition persists or gets worse.
- Do not use for longer than 1 week unless directed by a doctor.
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center right away.
- Tear open pouch and use as part of your daily cleansing routine.
- Clean the affected area.
- Apply this product on the area 1 to 3 times daily.
- May be covered with a sterile bandage.
- If bandaged, let dry first.
Store at room temperature between 59-86oF (15-30oC). If contact with eyes occurs, flush eyes with water.
Purified water
Call 1-866-322-4634, weekdays between 8:00 am and 6:00 pm EST
| LORIS BZK
ANTISEPTIC WIPES
benzalkonium chloride swab |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - LernaPharm Loris Inc. (206940905) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LernaPharm Loris Inc. | 206940905 | manufacture(68356-126), label(68356-126) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.