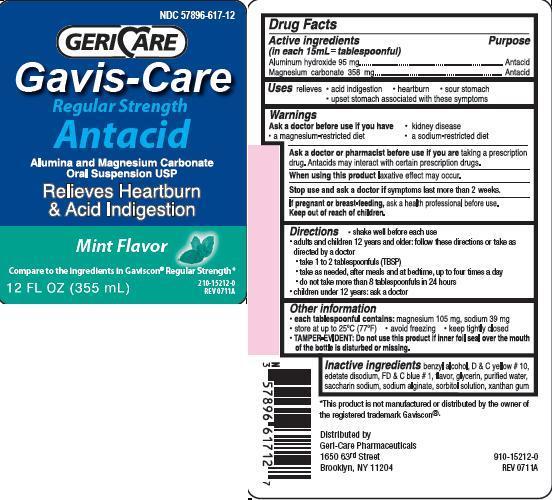
GAVIS-CARE ANTACID
Dosage form: suspension
Ingredients: ALUMINUM HYDROXIDE 95mg in 15mL, MAGNESIUM CARBONATE 358mg in 15mL
Labeler: Geri-Care Pharmaceuticals, Corp
NDC code: 57896-617
Medically reviewed by Drugs.com. Last updated on Dec 18, 2023.
Aluminum hydroxide 95 mg
Magnesium carbonate 358 mg
Antacid
relieves
- heartburn
- sour stomach
- acid indigestion
- upset stomach associated with these symptoms
Ask a doctor before use if you have
• kidney disease
• a magnesium-restricted diet
• a sodium-restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug.
Antacids may interact with certain prescription drugs.
When using this product laxative effect may occur.
Stop use and ask a doctor if symptoms last more than 2 weeks
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
• shake well before each use
• adults and children 12 years and older: follow these directions or take as directed by a doctor
• take 1 to 2 tablespoonfuls (TBSP)
• take as needed, after meals and at bedtime, up to four times a day
• do not take more than 8 tablespoonfuls in 24 hours
• children under 12 years: ask a doctor
•
each tablespoonful contains: magnesium 105 mg, sodium 39 mg
• store at up to 25C (77F)
• avoid freezing
• keep tightly closed
•
TAMPER-EVIDENT: Do not use this product if inner foil seal over the mouth of the bottle is disturbed or missing.
benzyl alcohol, D and C yellow no.10, edetate disodium, FD and C blue no.1, flavor, glycerin, purified water, saccharin sodium, sodium alginate, sorbitol solution, xanthan gum
| GAVIS-CARE ANTACID
aluminum hydroxide and magnesium carbonate suspension |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Geri-Care Pharmaceuticals, Corp (611196254) |
| Registrant - GCP Laboratories (965480861) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GCP Laboratories | 965480861 | manufacture(57896-617) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.