Medaclear
Dosage form: shampoo
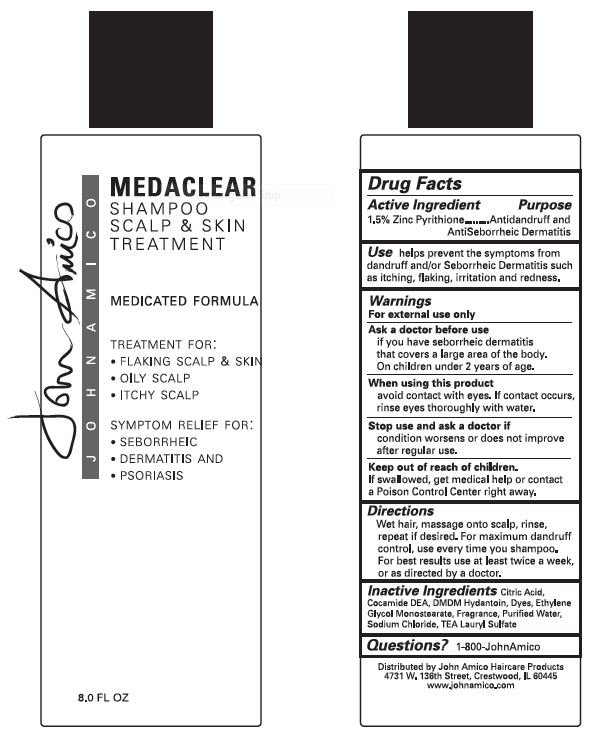
Ingredients: PYRITHIONE ZINC 15.0g in 1.0L
Labeler: Amico Educational Concepts, Inc.
NDC code: 51290-011
Medically reviewed by Drugs.com. Last updated on Dec 19, 2023.
Zinc Pyrithione 1.5%
Antidandruff and Antisebborrheic Dermatitis
helps prevent the symptoms from dandruff and/or Seborrheic Dermatitus such as itching, flaking, irritation and redness.
if you have seborrheic dermatitis that covers a large area of the body.
On children under 2 years of age.
avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
condition worsens or does not improve after regular use.
If swallowed, get medical help or contact a Poison Control Center right away.
Wet hair, massage into scalp, rinse, repeat if desired. For maximum dandruff control, use every time you shampoo. For best results, use twice a week, or as directed by a doctor.
Shampoo Scalp & Skin Treatment
Medicated Formula
Treatment for:
- Flaking Scalp & Skin
- Oily Scalp
- Itchy Scalp
Symptom Relief for:
- Seborrheic Dermatitus and Psoriasis
Citric Acid, Cocamide DEA, DMDM Hydantoin, Dyes, Ethylene Glycol Monostearate, Fragrance, Purified Water, Sodium Chloride, TEA Lauryl Sulfate
1-800-JohnAmico
John Amico Haircare Products
4731 W. 136th Street, Crestwood, IL 60445
www.johnamico.com
| MEDACLEAR
zinc pyrithione shampoo |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Amico Educational Concepts, Inc. (127642333) |
| Registrant - Continental Manufacturing Chemist, Inc. (005278007) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Continental Manufacturing Chemist, Inc. | 005278007 | MANUFACTURE(51290-011) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.