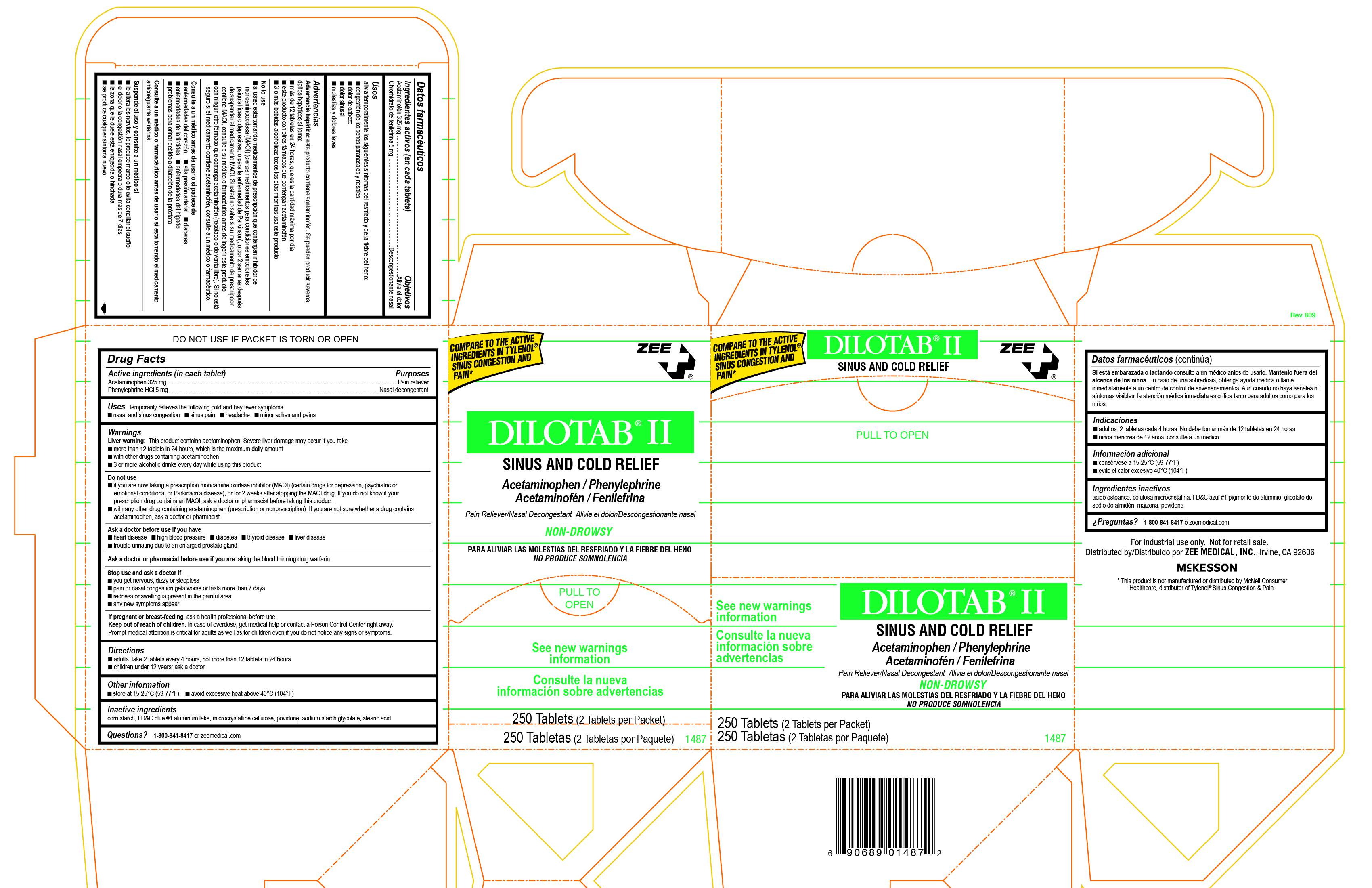
Dilotab II
Dosage form: tablet
Ingredients: ACETAMINOPHEN 325mg, PHENYLEPHRINE HYDROCHLORIDE 5mg
Labeler: Zee Medical Inc
NDC code: 35418-136
Medically reviewed by Drugs.com. Last updated on Oct 30, 2023.
Active Ingredient (in each tablet) Acetaminophen 325 mg, Phenylephrine HCL-Nasal Decongestant
Purpose-Pain Reliever Nasal Decongestant
Uses temporarily relieves the following cold and hay fever symptoms:
■ nasal and sinus congestion ■ sinus pain ■ headache ■ minor aches and pains
Warnings
Directions
■ adults: take 2 tablets every 4 hours, not more than 12 tablets in 24 hours
Children under 12 years, ask a doctor
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
■ more than 12 tablets in 24 hours, which is the maximum daily amount
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Do not use
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or
emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your
prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
■ heart disease ■ high blood pressure ■ diabetes ■ thyroid disease ■ liver disease
■ trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
Stop use and ask a doctor if
■ you get nervous, dizzy or sleepless
■ pain or nasal congestion gets worse or lasts more than 7 days
■ redness or swelling is present in the painful area
■ any new symptoms appear
If pregnant or breast-feeding baby, ask a health professional
before use
KEEP OUT OF REACH OF CHILDREN. In case of overdose,
get medical help or contact a Poison Control Center right away.
Prompt medical attention is critical for adults as well as for
children even if you do not notice any signs or symptoms.
Inactive Ingredients:corn starch, FDC blue 1 aluminum lake, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
| DILOTAB II
acetaminophen phenylephrine tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Zee Medical Inc (009645623) |
| Registrant - Ultra Seal Corporation (085752004) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ultratab Laboratories, Inc. | 151051757 | manufacture(35418-136) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ultra Seal Corporation | 085752004 | repack(35418-136) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.