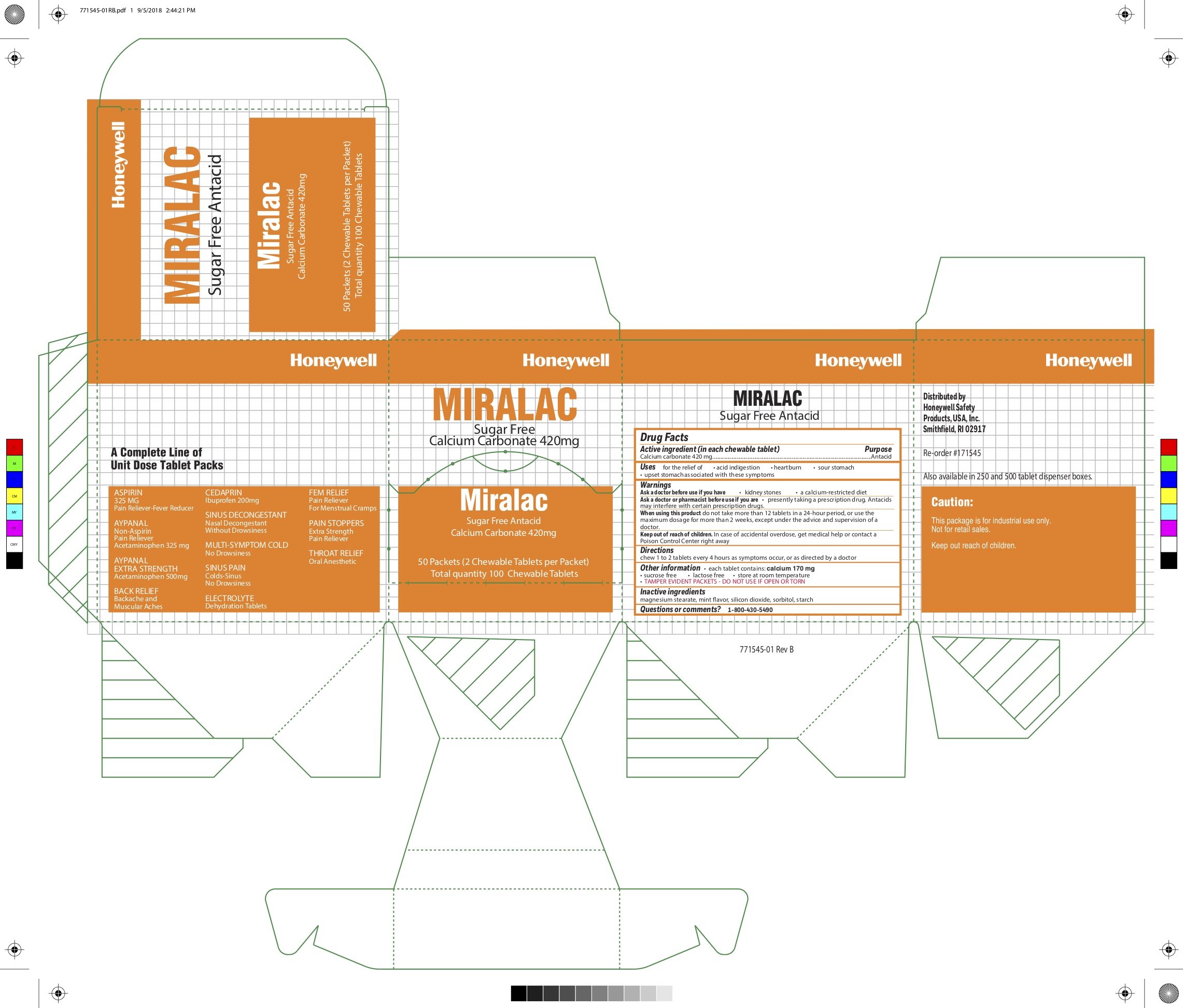
Miralac
Dosage form: tablet
Ingredients: CALCIUM CARBONATE 420mg
Labeler: Honeywell Safety Products USA, Inc
NDC code: 0498-0303
Medically reviewed by Drugs.com. Last updated on Jan 9, 2024.
Calcium carbonate 420 mg
Antacid
for the relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
- kidney stones
- calcium-restricted diet
presently taking a prescription drug. Antacids may interfere with certain prescription drugs
do not take more than 12 tablets in a 24- hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
chew 1 to 2 tablets every 4 hours as symptoms occur, or as directed by a doctor.
- each tablet contains: calcium 170 mg
- sucrose free
- lactose free
- store at room temperature
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
magnesium stearate, mint flavor, silicon dioxide, sorbitol, starch
1-800-430-5490
| MIRALAC
calcium carbonate tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (079287321) |
| Registrant - Honeywell Safety Products USA, Inc (079287321) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Honeywell Safety Products USA, Inc | 079287321 | repack(0498-0303) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.