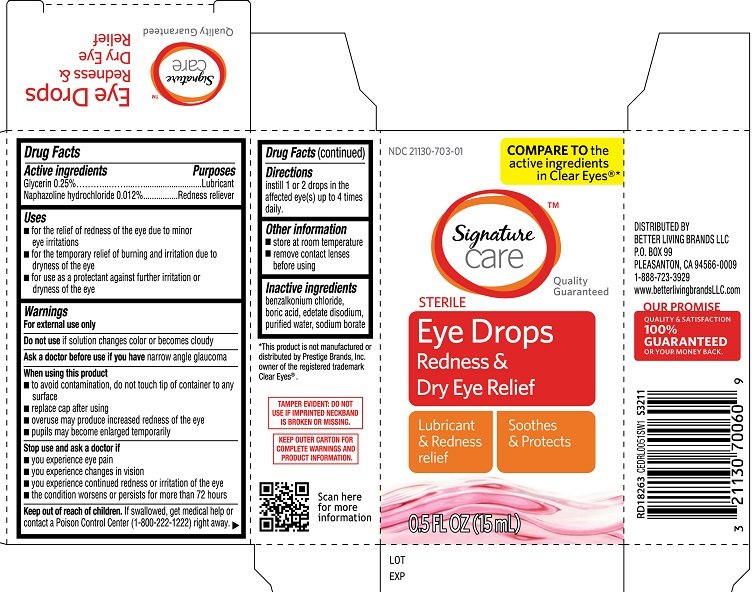
Signature Care Eye Drops Redness and Dry Eye Relief
Dosage form: liquid
Ingredients: GLYCERIN 0.25g in 100mL, NAPHAZOLINE HYDROCHLORIDE 0.012g in 100mL
Labeler: Better Living Brands LLC
NDC code: 21130-703
Medically reviewed by Drugs.com. Last updated on Jan 17, 2024.
Active ingredients
Glycerin 0.25%
Naphazoline hydrochloride 0.012%
Purposes
Glycerin Lubricant
Naphazoline hydrochloride Redness reliever
Uses
- for the relief of redness of the eye due to minor eye irritations
- for the temporary relief of burining and irritation due to dryness of the eye
- for use as a protectant against further irritation or dryness of the eye
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
Ask a doctor before use if you have
narrow angle glaucoma
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
- overuse may produce increased redness of the eye
- pupils may become enlarged temporarily
Stop use and ask a doctor if
- you experience eye pain
- changes in vision occur
- redness or irritation of the eye lasts
- condition worsens
- symptoms last for more than 72 hours
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Instill 1 or 2 drops in the affected eye(s) up to 4 times daily
Other information
- store at room temperature
- remove contact lenses before using
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate
| SIGNATURE CARE EYE DROPS REDNESS AND DRY EYE RELIEF
glycerin, naphazoline hydrochloride liquid |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - Better Living Brands LLC (009137209) |
| Registrant - KC Pharmaceuticals, Inc. (174450460) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| KC Pharmaceuticals, Inc. | 174450460 | manufacture(21130-703), pack(21130-703), label(21130-703) | |
Document Id: ee7b725a-e5d8-4d1f-b46e-04bfba718700
Set id: 901f3bbe-e1bb-4e44-879c-711e59813a7f
Version: 2
Effective Time: 20190127
Better Living Brands LLC
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.