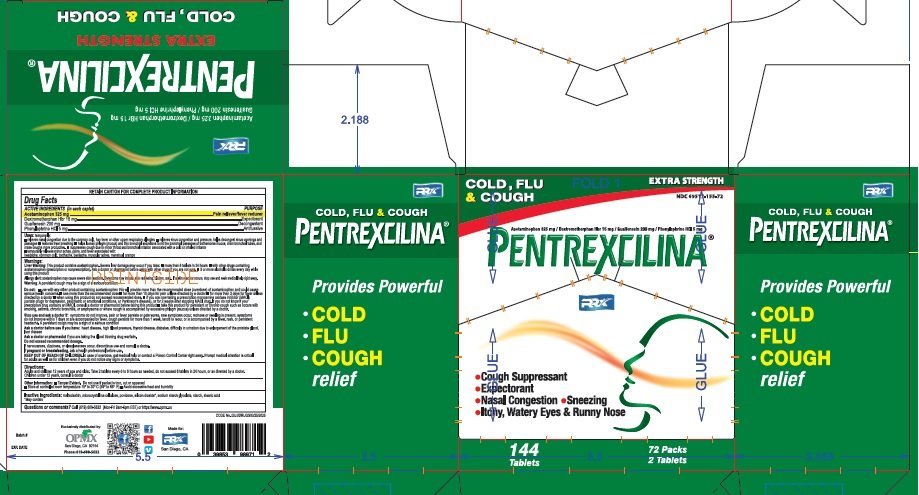
PENTREXCILINA
Dosage form: tablet
Ingredients: ACETAMINOPHEN 325mg, PHENYLEPHRINE HYDROCHLORIDE 5mg, DEXTROMETHORPHAN HYDROBROMIDE 15mg
Labeler: Healthlife of USA
NDC code: 69517-155
Medically reviewed by Drugs.com. Last updated on Nov 27, 2023.
Active Ingredients (in each tablet)
Acetaminophen USP 325mg.........................................................Pain reliever/fever reducer
Guaifenesin USP 200mg..............................................................Expectorant
Phenylephrine HCL USP 5mg........................................................Decongestant
Dextromethorphan HBr USP 15mg................................................Antitussive
Pain reliever/ fever reducer
Expectorant
Decongestant
Antitussive
Temporarily
- Relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- Relieves sinus congestion and pressure, helps decongest sinus openings and passages
- Restores freer breathing
- Helps loosen bothersome mucus, drain bronchial tubes, and make coughs more productive
- Suppresses cough due to minor throat and bronchial irritation associated with a cold or inhaled irritants
- Temporarily relieves minor aches, pains and fever associated with: headache, common cold, toothache, backache, muscular aches, menstrual cramps
This product contains acetaminophen. Severe liver damage may occur if you take:
- More than 8 tablets in 24 hours
- With other drugs containing acetaminophen (prescription or nonprescription). Ask a doctor or pharmacist before using with other drugs if you are not sure
- 3 or more alcoholic drinks every day while using this product
Acetaminophen may cause severe skin reactions. Symproms may include: skin reddening, blisters, rash if a skin reaction occurs, stop use and seek medical help right away.
Warning: A persistent cough may be a sign of a serious condition
- use with any other product containing acetaminophen this will provide more than the recommended dose (overdose) of acetaminophen and cold cause serious health concerns.
- use more than the recommended dose
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
- when using this product do not exceed recommended dose
- if you are now taking a presription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping MAOI drug. If you do not know if your prescription drug contains an MAOI, consult a doctor or pharmacist before taking this product
- take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema or where cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.
symptoms do not improve, pain or fever persists or gets worse, new symptoms occur, redness or swelling is present, symptoms do not improve within 7 days or are accompanied by fever, cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition
heart disease, high blood pressure, thyroid disease, diabetes, difficulty in urination due to enlargement of the prostate gland, liver disease
taking the blood thinning drug warfarin.
if nervousness, dizziness, or sleeplessness occur, discontinue use consult a doctor.
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not nnotice any signs or symptoms
Adults and children 12 years of age and older: Take 2 tablets every 6 to 8 hours as needed, do not exceed 8 tablets in 24 hours, or as directed by a doctor. Children under 12 years, consult a doctor
- Tamper evident. Do not use if packet is torn, cut or opened
- Store at controlled room temperature 15° to 30°C (59° to 86°F)
- Avoid excessive heat and humidity
maltodextrin, microcrystalline cellulose, povidone, silicon dioxide*, sodium starch glycolate, starch, stearic acid
(*may contain)
Call: 619-600-5632 (Mon-Fri 9am - 5pm EST)
| PENTREXCILINA
acetaminophen, phenylephrine hydrochloride, dextromethorphan hydrobromide tablet |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - Healthlife of USA (079656178) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Centurion Laboratories Pvt Ltd | 873229784 | manufacture(69517-155), analysis(69517-155), pack(69517-155) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.