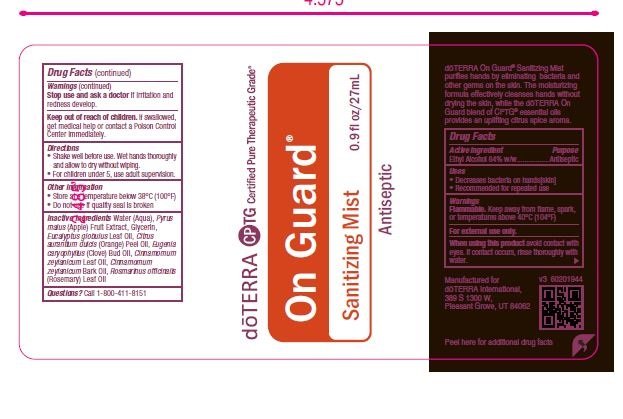
On Guard Sanitizing Mist
Dosage form: spray
Ingredients: ALCOHOL 70mL in 100mL
Labeler: doTERRA International, LLC
NDC code: 71630-718
Medically reviewed by Drugs.com. Last updated on Jan 24, 2024.
Uses
• Decreases bacteria on hands[skin]
• Recommended for repeated use
When using this product avoid contact with
eyes. If contact occurs, rinse thoroughly with
water.
Stop use and ask a doctor if irritation and
redness develop
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Other information
• Store at a temperature below 38°C (100°F)
• Do not use if quality seal is broken
Inactive ingredients: Water (Aqua), Pyrus
malus (Apple) Fruit Extract, Glycerin,
Eucalyptus Globulus Leaf Oil, Citrus
aurantium dulcis (Orange) Peel Oil, Eugenia
caryophyllus (Clove) Bud Oil, Cinnamomum
zeylanicum Leaf Oil, Cinnamomum
zeylanicum Bark Oil, Rosmarinus officinalis
(Rosemary) Leaf Oil

Questions? Call 1-800-411-8151
Active ingredient Purpose
Alcohol 64% w/w.........................Antiseptic
Directions
• Wet hands thoroughly and allow to dry
without wiping.
• For children under 5, use adult supervision.
| ON GUARD SANITIZING MIST
alcohol spray |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - doTERRA International, LLC (832274935) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.