Hand Sanitizer Gift Set 3-pack
Dosage form: kit
Ingredients: ALCOHOL 62mL in 100mL
Labeler: Papermates, Inc. DBA Noteworthy
NDC code: 75997-030
Medically reviewed by Drugs.com. Last updated on Jun 26, 2023.
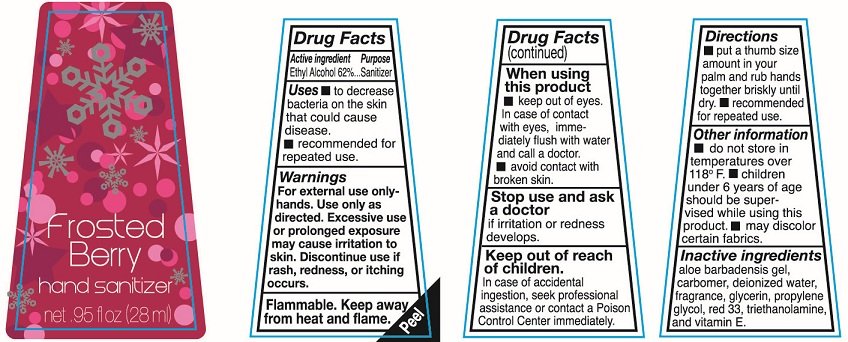
Ethyl Alcohol 62%
Sanitizer
To decrease bacteria on the skin that could cause disease.
recommended for repeated use
For external use only-hands. Use only as directed. Excessive use or prolonged exposure may cause irritation to skin. Discontinue use if rash redness or itching occurs
Flammable. keep away from heat and flame.
keep out of eyes. In case of contact with eyes immediately flush with water and call a doctor
avoid contact with broken skin.
Stop use and ask a doctor if irritation or redness develops.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
put a thumb size amount in your palm and rub hands together briskly until dry. recommended for repeated use.
do not store in temperatures over 118F.
Children under 6 years of age should be supervised while using this product.
may discolor certain fabrics.
aloe barbadensis gel, blue 1, carbomer, deionized water, fragrance, glycerin, propylene glycol, red 33, triethanolamine and vitamin E
Hand sanitizer
net .95fl oz (28ml)
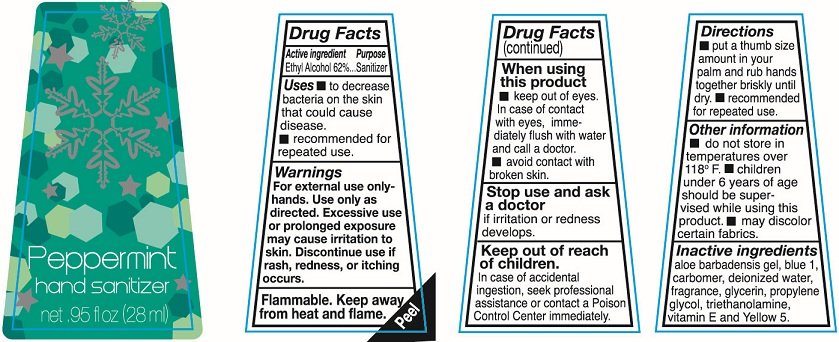
Ethyl Alcohol 62%
Sanitizer
To decrease bacteria on the skin that could cause disease.
recommended for repeated use
For external use only-hands. Use only as directed. Excessive use or prolonged exposure may cause irritation to skin. Discontinue use if rash redness or itching occurs
Flammable. keep away from heat and flame.
keep out of eyes. In case of contact with eyes immediately flush with water and call a doctor
avoid contact with broken skin.
Stop use and ask a doctor if irritation or redness develops.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
put a thumb size amount in your palm and rub hands together briskly until dry. recommended for repeated use.
do not store in temperatures over 118F.
Children under 6 years of age should be supervised while using this product.
may discolor certain fabrics.
aloe barbadensis gel, blue 1, carbomer, deionized water, fragrance, glycerin, propylene glycol, triethanolamine, vitamin E, and Yellow 5
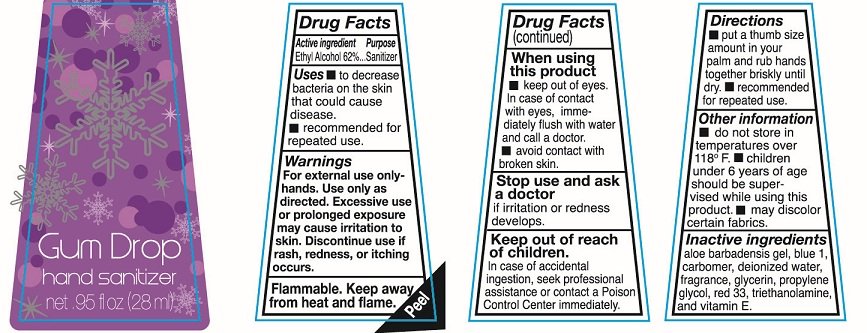
Ethyl Alcohol 62%
Sanitizer
To decrease bacteria on the skin that could cause disease.
recommended for repeated use
For external use only-hands. Use only as directed. Excessive use or prolonged exposure may cause irritation to skin. Discontinue use if rash redness or itching occurs
Flammable. keep away from heat and flame.
keep out of eyes. In case of contact with eyes immediately flush with water and call a doctor
avoid contact with broken skin.
Stop use and ask a doctor if irritation or redness develops.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
put a thumb size amount in your palm and rub hands together briskly until dry. recommended for repeated use.
do not store in temperatures over 118F.
Children under 6 years of age should be supervised while using this product.
may discolor certain fabrics.
aloe barbadensis gel, carbomer, deionized water, fragrance, glycerin, propylene glycol, red 33, triethanolamine and vitamin E
Hand sanitizer
net .95fl oz (28ml)
Hand sanitizer
net .95fl oz (28ml)
| HAND SANITIZER GIFT SET
3-PACK
alcohol kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Papermates, Inc. DBA Noteworthy (038734620) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.