Profend Nasal Decolonization
Dosage form: swab
Ingredients: POVIDONE-IODINE 10mg in 1mL
Labeler: Professional Disposables International, Inc.
NDC code: 10819-3888
Medically reviewed by Drugs.com. Last updated on Nov 6, 2023.
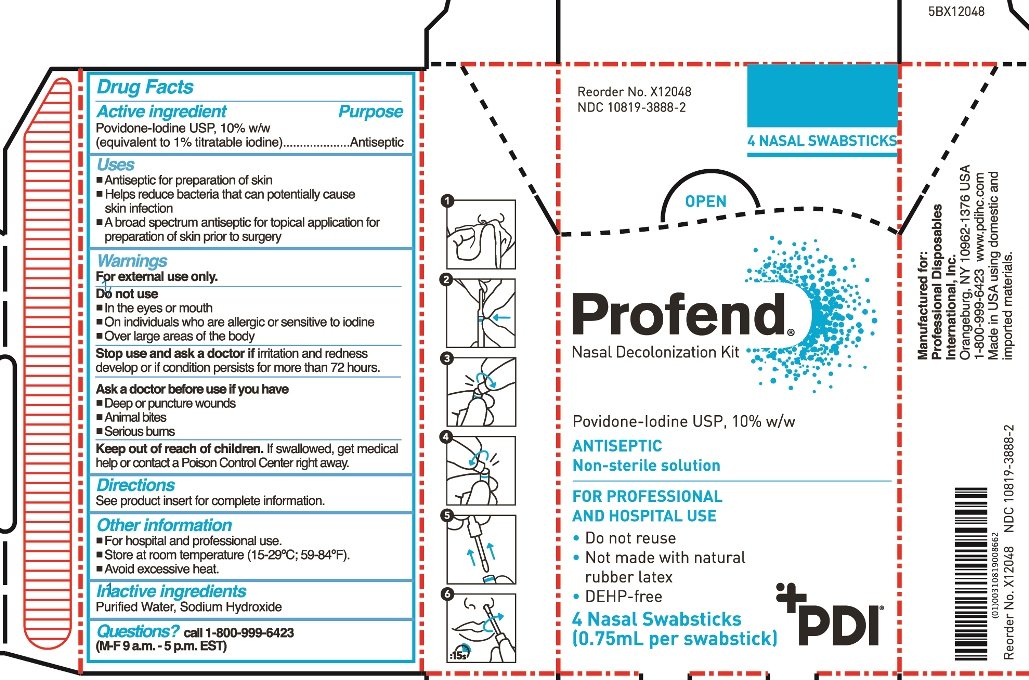
Povidone-Iodine USP 10% w/w (equivalent to 1.0% titratable iodine)
Antiseptic
- Antiseptic for preparation of skin
- Helps reduce bacteria that can potentially cause skin infection
- A broad spectrum antiseptic for topical application for preparation of skin prior to surgery
For external use only
Do not use
- in the eyes or mouth
- on individuals who are allergic or sensitive to iodine
- over large areas of the body
Stop use and ask doctor if irritation and redness develop or if condition persists for more than 72 hours.
Ask a doctor before use if you have
- Deep or puncture wounds
- animal bites
- serious burns
If swallowed, get medical help or contact a Poison Control Center right away.
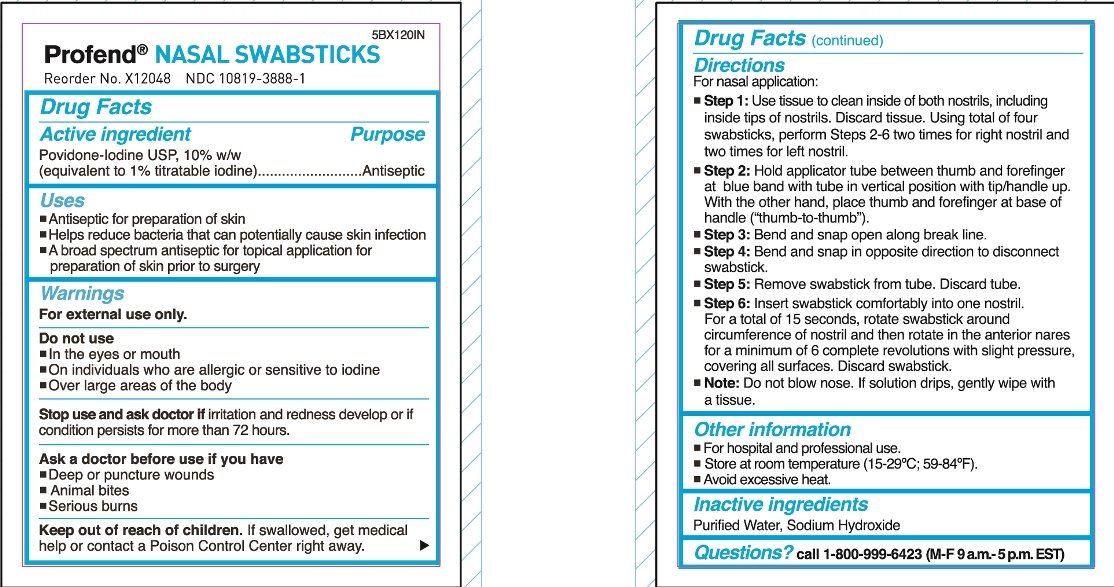
For nasal application:
Step 1: Use tissue to clean inside of both nostrils, including inside tips of nostrils. Discard tissue.
Using total of four swabsticks, perform Steps 2-6 two times for right nostril and two times for left nostril.
Step 2: Hold applicator tube between thumb and forefinger at blue band with tube in vertical position with tip/handle up. With the other hand, place thumb and forefinger at base of handle (“thumb-to-thumb”).
Step 3: Bend and snap open along break line.
Step 4: Bend and snap in opposite direction to disconnect swabstick.
Step 5: Remove swabstick from tube. Discard tube.
Step 6: Insert swabstick comfortably into one nostril. For a total of 15 seconds, rotate swabstick around circumference of nostril and then rotate in the anterior nares for a minimum of 6 complete revolutions with slight pressure, covering all surfaces. Discard swabstick.
Note: Do not blow nose. If solution drips, gently wipe with a tissue.
- For hospital and professional use
- Store at room temperature (15-29 oC; 59-84 oF)
- Avoid excessive heat
Purified Water, Sodium Hydroxide
call 1-800-999-6423 (M-F 9 a.m.-5 p.m. EST)
| PROFEND NASAL DECOLONIZATION
povidone iodine usp, 10% w/w swab |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Professional Disposables International, Inc. (800777117) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Professional Disposables International, Inc. | 800777117 | manufacture(10819-3888) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.