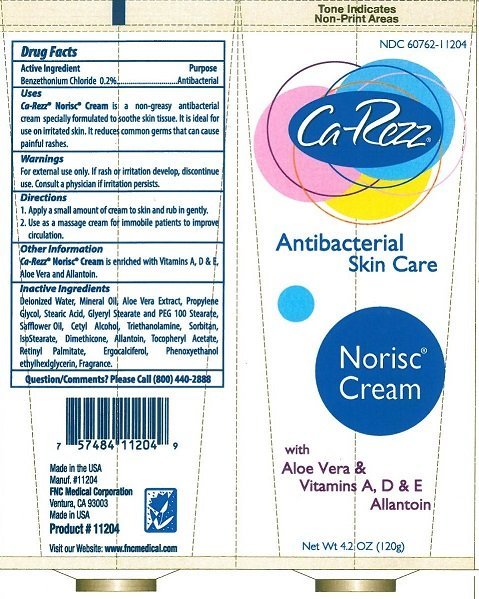
CA-REZZ NORISC ANTIBACTERIAL SKIN CARE
Dosage form: cream
Ingredients: BENZETHONIUM CHLORIDE 0.2g in 100g
Labeler: FNC MEDICAL CORPORATION
NDC code: 60762-112
Medically reviewed by Drugs.com. Last updated on Jan 26, 2024.
BENZETHONIUM CHLORIDE 0.2%
ANTIBACTERIAL
CA-REZZ NORSIC CREAM IS A NON-GREASY ANTIBACTERIAL CREAM SPECIALLY FORMULATED TO SOOTHE SKIN TISSUE. IT IS IDEAL FOR USE ON IRRITATED SKIN. IT REDUCES COMMON GERMS THAT CAN CAUSE PAINFUL RASHES.
FOR EXTERNAL USE ONLY. IF RASH OR IRRITATION DEVELOP, DISCONTINUE USE. CONSULT A PHYSICIAN IF IRRITATION PERSISTS.
KEEP OUT OF REACH OF CHILDREN.
- APPLY A SMALL AMOUNT OF CREAM TO SKIN AND RUB GENTLY.
- USE AS A MASSAGE CREAM FOR IMMOBILE PATIENTS TO IMPROVE CIRCULATION.
CA-REZZ NORSIC CREAM IS ENRICHED WITH VITAMINS A, D & E, ALOE VERA AND ALLANTOIN.
DEIONIZED WATER, MINERAL OIL, ALOE VERA EXTRACT, PROPYLENE GLYCOL, STEARIC ACID, GLYCERYL STEARATE AND PEG 100 STEARATE, SAFFLOWER OIL, CETYL ALCOHOL, TRIETHANOLAMINE, SORBITAN, ISOSTEARATE, DIMETHICONE, ALLANTOIN, TOCOPHERYL ACETATE, RETINYL PALMITATE, ERGOCALCIFEROL, PHENOXYETHANOL, ETHYLHEXYLGLYCERIN, FRAGRANCE.
PLEASE CALL (800) 440-2888
| CA-REZZ NORISC
ANTIBACTERIAL SKIN CARE
benzethonium chloride cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - FNC MEDICAL CORPORATION (849207519) |
| Registrant - FNC MEDICAL CORPORATION (849207519) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| FNC MEDICAL CORPORATION | 849207519 | label(60762-112), manufacture(60762-112) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.