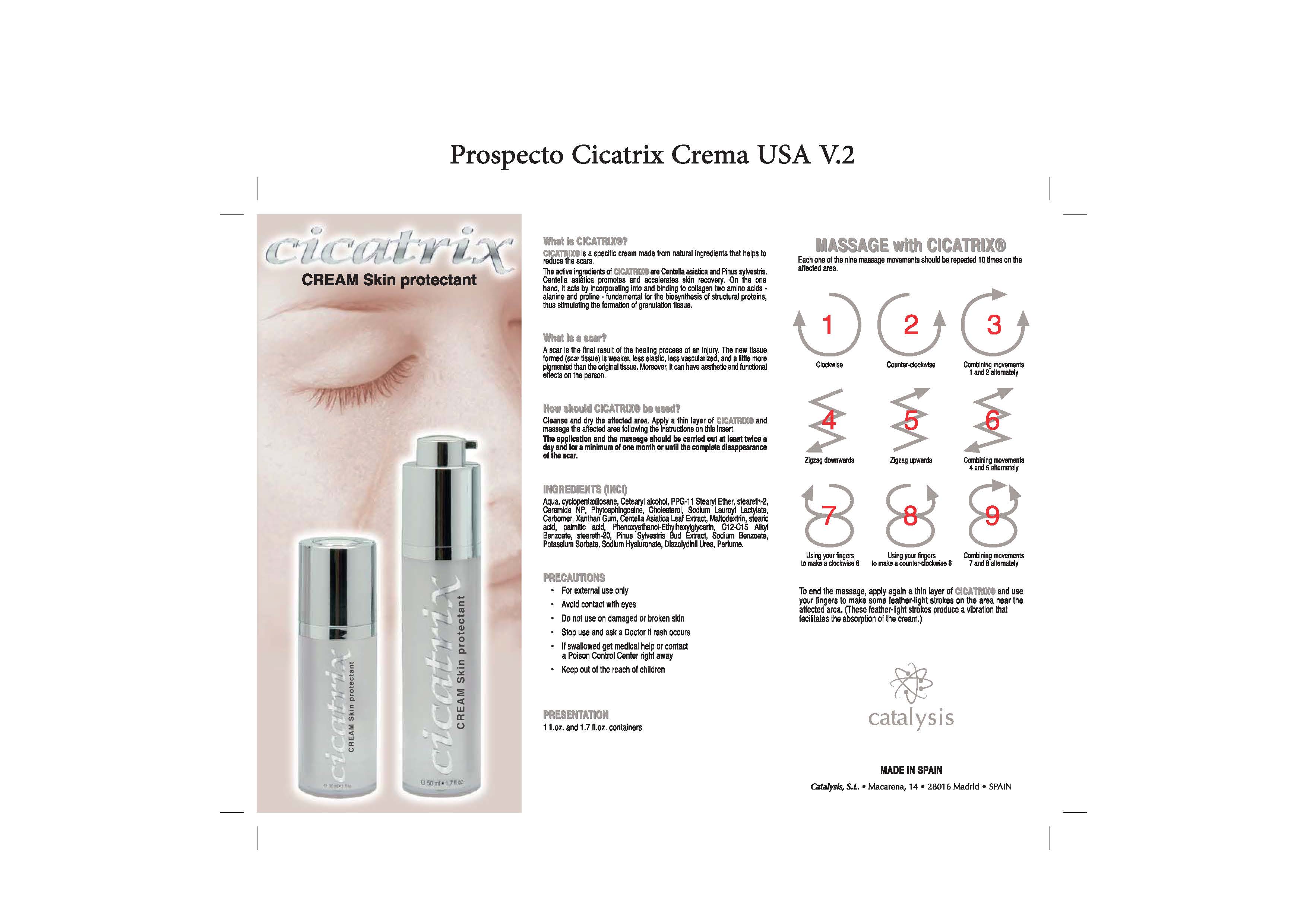
Cicatrix Skin Protectant
Dosage form: cream
Ingredients: GLYCERIN 3mg in 1mL
Labeler: Catalysis, SL
NDC code: 64539-011
Medically reviewed by Drugs.com. Last updated on Jan 18, 2024.
For external use only.
Do not use on damaged or broken skin.
When using this product keep our of the eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children
Children under 6 months: as a doctor.
Stop use and ask a doctor if rash occurs
Children under 6 months: ask a doctor
Keep out of reach of children
+ 34 M-F 9:00 am to 5:00 pm
. keep the product in a cool and dry place
. clean and dry the affected area.
. apply the product and massage the affected area
. follow the instructions on the insert
. apply at least twice a day
- clean and dry the affected area.
- apply the product and massage the affected area
- follow the instructions on the insert
- apply at least twice a day
. helps to reduce the scars and marks in t he skin
Aqua, Cyclopentasiloxane, Cetearyl Alcohol, PPG-11 Stearyl Ether, Steareth-2, Ceramide 3, Ceramide 6II, Ceramide 1, Phytosphingosine, Cholesterol, Sodium Lauroyl Lactylate, Carbomer, Xanthan Gum, Centella Asiatica Leaf Extract, Maltodextrin, Stearic Acid, Palmitic Acid, Phenoxyethanol, Ethylhexylglycerin, C12-C15 Alkyl Benzoate, Steareth-20, Pinus Sylvestris Bud Extract, Sodium Benzoate, Potassium Sorbate, Sodium Hyaluronate, Carbomer, Diazolydinil Urea, Parfum( coumarin, hexyl cinnamal,linalool,d-limonene/dipentene/l-limonene,Geraniol,hydroxycitronellal), Sodium Hydroxide
- helps to reduce the scars and marks in t he skin
| CICATRIX SKIN PROTECTANT
glycerin cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Catalysis, SL (862795119) |
| Registrant - Catalysis, SL (862795119) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Catalysis, SL | 862795119 | manufacture(64539-011) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.